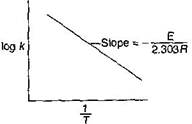
A) \[\log \,k\,vs\frac{1}{T}\]
B) \[\log \,k\,vs\,\frac{1}{\log \,T}\]
C) \[k\,vs\,T\]
D) \[k\,vs\frac{1}{\log \,T}\]
Correct Answer: A
Solution :
| [a] Arrhenius equation \[k=A{{e}^{-E/RT}}\] |
| \[\ln \,k=\ln \,A-\frac{E}{RT}\] |
| or \[\log \,k=\log \,A-\frac{E}{2.303\,RT}\] |
| Hence, E is calculated with the help of slope of following |
 |
You need to login to perform this action.
You will be redirected in
3 sec
