A) \[Ni{{(CO)}_{4}}\]-Tetrahedral, paramagnetic
B) \[Ni(CN)_{4}^{2-}\]-Square planar, diamagnetic
C) \[Ni{{(CO)}_{4}}\]-Tetrahedral, diamagnetic
D) \[{{[Ni\,{{(Cl)}_{4}}]}^{2}}\]-Tetrahedral, paramagnetic
Correct Answer: C
Solution :
| [c] In \[Ni{{(CO)}_{4}}\], Ni has zero oxidation number |
| So, \[_{28}Ni\text{ }=1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{8}},\text{ }4{{s}^{2}}\] |
| In excited state and during the formation of \[Ni{{(CO)}_{4}}\to \] |
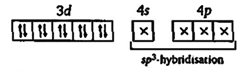 |
| Hence in it, no unpaired electron is present. So it shows the property of diamagnetism and tetrahedral structure. |
You need to login to perform this action.
You will be redirected in
3 sec
