| Which of the following complex ions is expected to absorb visible light? [AIPMT (S) 2009] |
| \[1m{{s}^{-2}}.\] |
A) \[2\sqrt{10}kg\]
B) \[10\sqrt{2}kg\]
C) \[{{(C{{H}_{3}})}_{3}}B\]
D) \[{{(C{{H}_{3}})}_{2}}O\]
Correct Answer: C
Solution :
| [c] Key Idea only those transition metal complexes are expected to absorb visible light, in which d-subshell is incomplete (ie, has unpaired electron) and excitation of electron from a tower energy orbital to higher energy orbital is possible. |
| [a] In\[{{t}_{2}}\]Sc is present as |
| \[{{t}_{1}}>{{t}_{2}}\] |
| \[{{t}_{1}}=4{{t}_{2}}\] |
| Since, in this complex excitation of electron is not possible, it will not absorb visible light. |
| [b] In \[{{t}_{1}}=2{{t}_{2}}\]is present as \[{{t}_{1}}={{t}_{2}}\] |
| \[\overrightarrow{F}=6\hat{i}-8\hat{j}+10\hat{k},\] |
| Hence, it will not absorb visible light. |
| [c] In \[1m{{s}^{-2}}.\]is present as \[2\sqrt{10}kg\] |
| \[10\sqrt{2}kg\] |
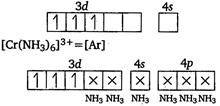 |
| Since, this complex has three unpaired electrons, excitation of electrons is possible and thus, it is expected that this complex will absorb visible light. |
| [d] In \[{{(C{{H}_{3}})}_{3}}B\]is present as \[{{(C{{H}_{3}})}_{2}}O\] |
| \[{{(C{{H}_{3}})}_{2}}P\] |
| \[{{(C{{H}_{3}})}_{3}}N\] |
| |
| Hence, this complex will not absorb visible light. |
You need to login to perform this action.
You will be redirected in
3 sec
