A) \[{{\text{ }\!\![\!\!\text{ Ni(CN}{{\text{)}}_{\text{4}}}\text{ }\!\!]\!\!\text{ }}^{\text{2-}}}\]
B) \[{{\text{ }\!\![\!\!\text{ Cr(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}\]
C) \[{{\text{ }\!\![\!\!\text{ Fe(}{{\text{H}}_{2}}\text{O}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
D) \[{{\text{ }\!\![\!\!\text{ Ni(}{{\text{H}}_{2}}\text{O}{{\text{)}}_{6}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
Correct Answer: A
Solution :
| [a] Key Idea For the absorption of visible light, presence of unpaired d-electrons is the necessity. |
| [a] In \[{{[Ni{{(CN)}_{4}}]}^{2-}},Ni\] is present as \[N{{i}^{2+}}.\] |
| \[N{{i}^{2+}}=[Ar]\,3{{d}^{8}}4{{s}^{0}}\] |
| \[\therefore {{[Ni{{(CN)}_{4}}]}^{2-}}\] |
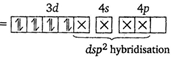 |
| (Pairing occurs because \[\text{C}{{\text{N}}^{-}}\]is a strong field ligand). |
| Since, in \[{{[Ni{{(CN)}_{4}}]}^{2-}},\]no unpaired electron is present in d-orbitals, it does not absorb visible light. |
| [b] In\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]is present as \[C{{r}^{3+}}\] \[C{{r}^{3+}}=[Ar]3{{d}^{3}}4{{s}^{0}}\](Three unpaired electrons) |
| [c] In \[{{[Fe{{({{H}_{2}}O)}_{6}}]}^{2+}},\]Fe is present as \[F{{e}^{2+}}.\] |
| \[F{{e}^{2+}}=[Ar]3{{d}^{6}}4{{s}^{0}}\] (Four unpaired electrons) |
| [d] In \[{{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}},Ni\]is present as \[N{{i}^{2+}}.\] |
| \[N{{i}^{2+}}=[Ar]3{{d}^{8}}4{{s}^{o}}\](Two unpaired electrons) |
| The complexes given in option , [c], [d] have unpaired electrons, thus absorb visible light. |
You need to login to perform this action.
You will be redirected in
3 sec
