A) \[{{\text{ }\!\![\!\!\text{ Ni(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
B) \[{{\text{ }\!\![\!\!\text{ Zn(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{2+}}}\]
C) \[{{\text{ }\!\![\!\!\text{ Cr(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}\]
D) \[{{\text{ }\!\![\!\!\text{ Co(N}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{6}}}\text{ }\!\!]\!\!\text{ }}^{\text{3+}}}\]
Correct Answer: A
Solution :
| [a] Outer orbital complex utilizes \[nd\] orbitals for bonding and exhibit paramagnetic behaviour, only if there present unpaired electrons. |
| [a] In \[{{[Ni{{(N{{H}_{3}})}_{6}}]}^{2+}}:\] \[N{{i}^{2+}}=[Ar]3{{d}^{8}}4{{s}^{o}}\] |
| |
| \[{{[Ni{{(N{{H}_{3}})}_{6}}]}^{2+}}=\] |
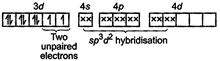 |
| So, this is an outer orbital complex having paramagnetic character. |
| [b] In\[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}:\]\[Z{{n}^{2+}}=[Ar]\,3{{d}^{10}}\] |
| |
| \[{{[Zn{{(N{{H}_{3}})}_{6}}]}^{2+}}=\] |
| |
| Thus, it is also an outer orbital complex but it |
| is diamagnetic as all the electrons are paired. |
| [c] In\[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}},\]\[C{{r}^{3+}}=[Ar]\,3{{d}^{3}}\] |
| \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}=\] |
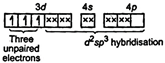 |
| Because of the involvement of (n - 1) d, i. e., 3d orbital-in hybridization, it is an inner orbital complex. Its nature is paramagnetic because of the presence of three unpaired electrons. |
| [d] In \[{{[CO{{(N{{H}_{3}})}_{6}}]}^{3+}}:\]\[C{{o}^{3+}}=[Ar]\,3{{d}^{6}}\] |
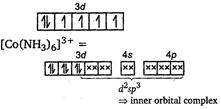 |
| As all the electrons are paired, it is a diamagnetic complex. |
You need to login to perform this action.
You will be redirected in
3 sec
