A) It is \[ds{{p}^{2}}\]hybridised and square planar
B) It is \[s{{p}^{3}}{{d}^{2}}\]hybridised and octahedral
C) It is \[s{{p}^{3}}{{d}^{2}}\] hybridised and tetrahedral
D) It is\[{{d}^{2}}s{{p}^{3}}\] hybridised and octahedral
Correct Answer: D
Solution :
| [d] \[{{[Mn{{(CN)}_{6}}]}^{3-}}\] |
| \[Mn(III)=[Ar]3{{d}^{4}}\] |
| \[C{{N}^{-}}\]being strong field ligand forces pairing of electrons |
| This gives\[t_{2}^{4}e_{g}^{0}\] |
| \[\therefore \]Mn(III) = [Ar] |
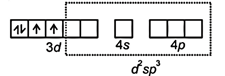 |
| ∵ Coordination number of Mn = 6 |
| \[\therefore \]Structure = octahedral |
| \[{{[Mn{{(CN)}_{6}}]}^{3-}}=\] |
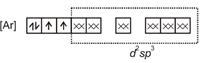 |
You need to login to perform this action.
You will be redirected in
3 sec
