A) \[\text{C}{{\text{H}}_{\text{3}}}\text{CH}\left( \text{OH} \right)\text{C}{{\text{H}}_{\text{3}}}\]
B) \[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CH}\left( \text{OH} \right)\text{C}{{\text{H}}_{\text{3}}}\]
C) \[\text{C}{{\text{H}}_{\text{3}}}\text{OH}\]
D) \[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{OH}\]
Correct Answer: C
Solution :
[c] An organic compound form yellow precipitate of iodoform with \[{{I}_{2}}\] in presence of alkali, if it has\[\text{C}{{\text{H}}_{\text{3}}}\text{CO}\]group directly or it has 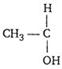 group group |
| \[\text{C}{{\text{H}}_{\text{3}}}\text{CH}\left( \text{OH} \right)\text{C}{{\text{H}}_{\text{3}}}+{{\text{l}}_{\text{2}}}\text{ }\xrightarrow{\text{NaoH}}\text{ C}{{\text{H}}_{\text{3}}}\text{COC}{{\text{H}}_{\text{3}}}\]\[\begin{align} & \,\,\,\,\,\,\,\,\,\,+\text{ }2HI+3NaI+C{{H}_{3}}COONa+\underset{{}}{\mathop{3{{H}_{2}}O}}\, \\ & C{{H}_{3}}COC{{H}_{3}}+3{{I}_{2}}+4NaOH\xrightarrow{{}}\underset{\begin{smallmatrix} \text{yellow} \\ \text{ppt}\text{.} \end{smallmatrix}}{\mathop{CH{{I}_{3}}}}\,\downarrow \\ \end{align}\] |
| \[+\text{ }3NaI+C{{H}_{3}}COONa+3{{H}_{2}}O\] |
| \[C{{H}_{3}}C{{H}_{2}}CH\left( OH \right)\text{ }C{{H}_{3}}+{{I}_{2}}\xrightarrow[{}]{{}}\]\[\underset{Ethyl\,methyl\,ketone}{\mathop{C{{H}_{3}}C{{H}_{2}}\underset{\underset{O}{\mathop{||}}\,}{\mathop{C}}\,C{{H}_{3}}+2HI}}\,\] |
| It gives iodoform test. |
| \[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\underset{\underset{\text{O}}{\mathop{||}}\,}{\mathop{\text{C}}}\,\text{C}{{\text{H}}_{\text{3}}}+\text{3}{{\text{l}}_{\text{2}}}+\text{4NaOH }\to \]\[\underset{\text{Yellow}\,\text{ppt}\text{.}}{\mathop{\text{CH}{{\text{I}}_{\text{3}}}}}\,\downarrow +\text{3NaI+C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{COONa+3}{{\text{H}}_{\text{2}}}\text{O}\] |
| \[\text{C}{{\text{H}}_{\text{3}}}\text{OH}+{{\text{1}}_{\text{2}}}\text{ }\xrightarrow{{}}\text{ HCHO}+\text{2HI}\] |
| It does not have methyl ketonic group, so it does not give yellow ppt. with \[{{\text{I}}_{\text{2}}}\] in presence of alkali. |
| \[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{OH}+{{\text{1}}_{\text{2}}}\xrightarrow{\text{ }}\text{ C}{{\text{H}}_{\text{3}}}\underset{\underset{\text{O}}{\mathop{||}}\,}{\mathop{\text{C}}}\,\text{H}+\text{2HI}\] |
| It gives iodoform test. |
| \[\text{C}{{\text{H}}_{\text{3}}}\underset{\underset{\text{O}}{\mathop{||}}\,}{\mathop{\text{C}}}\,\text{H}+\text{3}{{\text{I}}_{\text{2}}}+\text{4NaOH }\xrightarrow{{}}\underset{\text{Yellow}\,\text{ppt}\text{.}}{\mathop{\text{CH}{{\text{l}}_{\text{3}}}}}\,\]\[+HCOONa+3NaI+2{{H}_{2}}O\] |
You need to login to perform this action.
You will be redirected in
3 sec
