A) \[C{{l}_{2}}{{O}_{7}}\] is an anhydride of perchloric acid
B) \[{{O}_{3}}\]molecule is bent
C) OFN is isoelectronic with \[N{{O}_{2}}\]
D) \[O{{F}_{2}}\]is an oxide of fluorine
Correct Answer: A
Solution :
| [a] \[C{{l}_{2}}{{O}_{7}}\] is an anhydride of perchloric acid |
| \[2HCl{{O}_{4}}\xrightarrow[-{{H}_{2}}O]{\Delta }C{{l}_{2}}{{O}_{7}}\] |
| [a] Shape of \[{{O}_{3}}\] molecule is bent. |
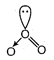 |
| [c] Number of electrons in ONF = 24 |
| Number of electrons in \[N{{O}_{2}}\] = 24 |
| \[\therefore \] ONF and \[N{{O}_{2}}\] both are isoelectronic. |
| [d] \[O{{F}_{2}}\] is a fluoride of oxygen because electronegativity of fluorine is more than that of oxygen. |
| \[O{{F}_{2}}\] = Oxygen difluoride |
You need to login to perform this action.
You will be redirected in
3 sec
