A) presence of one OH group and two PH bonds
B) high electron gain enthalpy of phosphorus
C) high oxidation state of phosphorus
D) presence of two OH groups and one PH bond
Correct Answer: A
Solution :
| [a] The oxy acid of phosphorus which contain PH bond act as a reducing agent or reductant. |
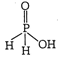 |
| In \[{{H}_{3}}P{{O}_{2}}\] oneOH group and two PH bonds are present. |
You need to login to perform this action.
You will be redirected in
3 sec
