A) \[\frac{2}{\sqrt{3}}a\]
B) \[\frac{4}{\sqrt{3}}a\]
C) \[\frac{\sqrt{3}}{4}a\]
D) \[\frac{\sqrt{3}}{2}a\]
Correct Answer: D
Solution :
| [d] In bcc, 2 atoms are present. One atom lie at the centre of cube while other lies at the corner of the cube. Hence, the distance between the body centered and one corner atom is half of the body diagonal, i.e., \[\frac{\sqrt{3}}{2}a.\] |
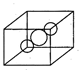 |
You need to login to perform this action.
You will be redirected in
3 sec
