| The following equilibrium constants are given: [AIPMT (S) 2007] |
| \[{{N}_{2}}+3{{H}_{2}}\,\rightleftharpoons \,2N{{H}_{3}}\,;\,{{K}_{1}}\] |
| \[{{N}_{2}}+{{O}_{2}}\,\,\rightleftharpoons \,2NO\,;\,{{K}_{2}}\] |
| \[{{H}_{2}}+1/2\,{{O}_{2}}\,\rightleftharpoons \,{{H}_{2}}O\,;\,{{K}_{3}}\] |
| The equilibrium constant for the oxidation of \[N{{H}_{3}}\] by oxygen to give NO is: |
A) \[{{K}_{2}}K_{3}^{3}/{{K}_{1}}\]
B) \[{{K}_{2}}\,K_{3}^{2}/{{K}_{1}}\]
C) \[K_{2}^{2}\,{{K}_{3}}/{{K}_{1}}\]
D) \[{{K}_{1}}\,{{K}_{2}}/{{K}_{3}}\]
Correct Answer: A
Solution :
| The required equation for the oxidation of NH3 by oxygen to give NO is : |
| |
| For this |
| For the equation |
| For the equation |
| For the equation |
| For getting the K we must do |
| |
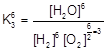 |
| |
| |
| |
You need to login to perform this action.
You will be redirected in
3 sec
