| From the following bond energies: [AIPMT (S) 2009] |
| \[H-H\] bond energy: 431.37 kJ \[mo{{l}^{-1}}\] |
| \[C=O\] bond energy: 606.10 kJ \[mo{{l}^{-1}}\] |
| \[C-C\] bond energy: 336.49 kJ \[mo{{l}^{-1}}\] |
| \[C-H\] bond energy: 410.50 kJ \[mo{{l}^{-1}}\] |
| Enthalpy for the reaction, |
| \[\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\,=\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\,+H-\xrightarrow{{}}H-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\,-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} H \\ | \end{smallmatrix}}{\mathop{C}}}\,-H\] will be |
A) \[1523.6\,kJ\,mo{{l}^{-1}}\]
B) \[-243.6\,kJ\,mo{{l}^{-1}}\]
C) \[-120.0\,kJ\,mo{{l}^{-1}}\]
D) \[553.0\,kJ\,mo{{l}^{-1}}\]
Correct Answer: C
Solution :
| Key Idea, Enthalpy of reaction |
| |
| For reaction, |
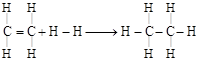 |
| |
| |
| |
| |
| |
You need to login to perform this action.
You will be redirected in
3 sec
