A) \[\Delta S=0\]
B) \[\Delta G=0\]
C) \[\Delta H=0\]
D) \[\Delta H=\Delta G=\Delta S=0\]
Correct Answer: C
Solution :
Energy profile diagram for a reaction is as From the figure, it is clear that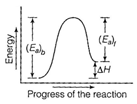 \[{{({{E}_{a}})}_{b}}={{({{E}_{a}})}_{t}}+\Delta H\] [Here \[{{({{E}_{a}})}_{b}}=\] activation energy of forward reaction]. If \[{{({{E}_{a}})}_{b}}={{({{E}_{a}})}_{t}}\] then \[\Delta H=0\]
\[{{({{E}_{a}})}_{b}}={{({{E}_{a}})}_{t}}+\Delta H\] [Here \[{{({{E}_{a}})}_{b}}=\] activation energy of forward reaction]. If \[{{({{E}_{a}})}_{b}}={{({{E}_{a}})}_{t}}\] then \[\Delta H=0\]
You need to login to perform this action.
You will be redirected in
3 sec
