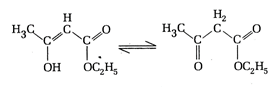
A) 18 sigma bonds and 2 pi-bonds
B) 16 sigma bonds and 1 pi-bond
C) 9 sigma bonds and 2 pi-bonds
D) 9 sigma bonds and 1 pi-bond
Correct Answer: A
Solution :
The enolic form of ethyl acetoacetate has 16 single bonds i.e. 16 \[\sigma \]-bonds and 2 double bonds i.e. 2 \[\sigma \]-bonds and 2 \[\pi \]-bonds. Hence, the given structure has 18 \[\sigma \]-bonds and 2\[\pi \]-bonds.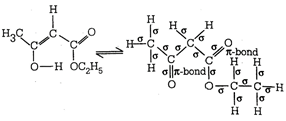
You need to login to perform this action.
You will be redirected in
3 sec
