A) \[ds{{p}^{2}}\]
B) \[s{{p}^{3}}\]
C) \[{{d}^{2}}s{{p}^{2}}\]
D) \[{{d}^{2}}s{{p}^{3}}\]
Correct Answer: A
Solution :
\[{{[Ni(CN)4]}^{2-}}\] Let oxidation state of Ni in \[{{[Ni(CN)4]}^{2-}}\]is x. \[\therefore \] \[x-4=2\] or x = 2 Now, \[N{{i}^{2+}}=[Ar],3{{d}^{8}},4{{s}^{0}}\]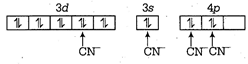 \[\therefore \] Hybridisation of \[{{[Ni{{(CN)}_{2}}]}^{2-}}\]is \[ds{{p}^{2}}\]
\[\therefore \] Hybridisation of \[{{[Ni{{(CN)}_{2}}]}^{2-}}\]is \[ds{{p}^{2}}\]
You need to login to perform this action.
You will be redirected in
3 sec
