A) \[{{C}_{2}}{{H}_{6}}\]
B) \[{{C}_{2}}{{H}_{4}}\]
C) \[BeC{{l}_{2}}\]
D) \[{{C}_{2}}{{H}_{2}}\]
Correct Answer: B
Solution :
[b] When there is\[3\sigma \]bonds, the hybridisation is \[s{{p}^{2}}\]. The structures of the given compounds are [a]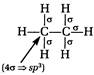 [b]
[b] 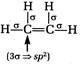 [c]
[c] You need to login to perform this action.
You will be redirected in
3 sec
