A) \[{{K}_{h}}=\frac{{{K}_{\omega }}}{{{K}_{a}}}\]
B) \[{{K}_{h}}=\frac{{{K}_{\omega }}}{{{K}_{a}}.{{K}_{b}}}\]
C) \[{{K}_{h}}=\frac{{{K}_{\omega }}}{{{K}_{h}}}\]
D) hydrolysis does not occur
Correct Answer: A
Solution :
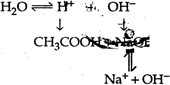 Solution will be basic The \[{{K}_{h}}\] value of basic solution will be \[{{K}_{h}}=\frac{{{K}_{\omega }}}{{{K}_{a}}}\]
Solution will be basic The \[{{K}_{h}}\] value of basic solution will be \[{{K}_{h}}=\frac{{{K}_{\omega }}}{{{K}_{a}}}\]
You need to login to perform this action.
You will be redirected in
3 sec
