A) \[{{120}^{o}}\] and \[{{90}^{o}}\]
B) \[{{60}^{o}}\] and \[{{90}^{o}}\]
C) \[{{60}^{o}}\] and \[{{120}^{o}}\]
D) \[{{120}^{o}}\] and \[{{30}^{o}}\]
Correct Answer: A
Solution :
\[Cl-P-Cl\]bond angles in \[PC{{l}_{5}}\] molecule are \[{{120}^{o}}\] and \[{{90}^{o}}\].\[PC{{l}_{5}}\] having \[s{{p}^{3}}d\] hybridised P atom (trigonal bipyramidal geometry) has two types of bonds; axial and equatorial. These two types of bond have different bond lengths 1, 2, 3 - equatorial bonds and 4, 5 axial bonds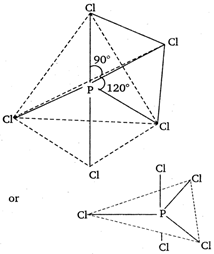 c
c
You need to login to perform this action.
You will be redirected in
3 sec
