A) zero order
B) first order
C) second order
D) third order
Correct Answer: B
Solution :
A graph between the log concentration, \[(\log C)\] of reactent and time t for the first order, reaction gives a straight line, whose slope is equal to \[-\frac{k}{2.303}\]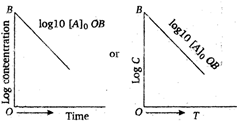 \[{{\log }_{10}}\,{{C}_{A}}=-\frac{kt}{2.303}+{{\log }_{10}}{{({{C}_{A}})}_{0}}\] Hence, the order of the above reaction is one.
\[{{\log }_{10}}\,{{C}_{A}}=-\frac{kt}{2.303}+{{\log }_{10}}{{({{C}_{A}})}_{0}}\] Hence, the order of the above reaction is one.
You need to login to perform this action.
You will be redirected in
3 sec
