A) \[s{{p}^{3}}d\]
B) \[s{{p}^{3}}\]
C) \[s{{p}^{3}}{{d}^{2}}\]
D) \[s{{p}^{3}}{{d}^{3}}\]
Correct Answer: A
Solution :
Key Idea: Write the valence shell configuration of iodine (I) to find the hybridisation type in \[\text{I}{{\text{F}}_{\text{3}}}\text{.}\]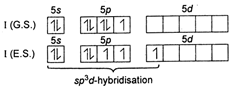 \[\because \] In \[\text{I}{{\text{F}}_{\text{3}}}\] three unpaired electrons are needed. \[\therefore \] \[\text{I}{{\text{F}}_{\text{3}}}\] has \[\text{s}{{\text{p}}^{3}}d\]hybridisation.
\[\because \] In \[\text{I}{{\text{F}}_{\text{3}}}\] three unpaired electrons are needed. \[\therefore \] \[\text{I}{{\text{F}}_{\text{3}}}\] has \[\text{s}{{\text{p}}^{3}}d\]hybridisation.
You need to login to perform this action.
You will be redirected in
3 sec
