A) \[{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{3}}}\]is dibasic and reducing
B) \[{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{3}}}\]is dibasic and non-reducing
C) \[{{\text{H}}_{3}}P{{O}_{3}}\]is tribasic and reducing
D) \[{{\text{H}}_{3}}P{{O}_{3}}\]is tribasic and non-reducing
Correct Answer: A
Solution :
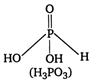 \[{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{3}}}\] is dibasic because it has two P?OH bonds and reducing due to the presence of one P?H bond.
\[{{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{3}}}\] is dibasic because it has two P?OH bonds and reducing due to the presence of one P?H bond.
You need to login to perform this action.
You will be redirected in
3 sec
