A) 1
B) 3
C) 4
D) 5
Correct Answer: A
Solution :
\[{{P}_{4}}{{O}_{10}}\]being a dehydrating agent, removes a molecule of water and forms anhydride of \[HN{{O}_{3}}.\] \[4HN{{O}_{3}}+{{P}_{4}}{{O}_{10}}\xrightarrow[{}]{{}}4HP{{O}_{3}}+2{{N}_{2}}{{O}_{5}}\] Resonating structures of \[{{N}_{2}}{{O}_{5}}\] are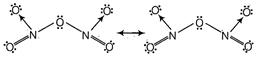
You need to login to perform this action.
You will be redirected in
3 sec
