A) \[Al{{F}_{3}}\]
B) \[N{{F}_{3}}\]
C) \[Cl{{F}_{3}}\]
D) \[B{{F}_{3}}\]
Correct Answer: C
Solution :
Cl in\[Cl{{F}_{3}}\]has\[s{{p}^{3}}d\]hybridisation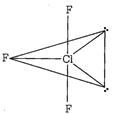 and possess two axial Cl-F bonds and one equatorial bond. Two lone pairs are at equatorial positions give rise to bent T' shape to \[Cl{{F}_{3}}.\]
and possess two axial Cl-F bonds and one equatorial bond. Two lone pairs are at equatorial positions give rise to bent T' shape to \[Cl{{F}_{3}}.\]
You need to login to perform this action.
You will be redirected in
3 sec
