question_answer 1) If the potential energy of two molecules is given by, \[u=\frac{A}{{{r}^{6}}}-\frac{{{B}^{2}}}{4A}\] then at equilibrium position, its potential energy is equal to:
A)
\[\frac{{{A}^{2}}}{4B}\]
done
clear
B)
\[\frac{{{B}^{2}}}{4A}\]
done
clear
C)
\[\frac{2B}{A}\]
done
clear
D)
\[-\frac{{{B}^{2}}}{4A}\]
done
clear
View Answer play_arrow
question_answer 2) A particle has angular momentum equal to A. The angular momentum becomes 4A after 4 sec. Find the torque applied:
A)
\[\frac{A}{4}\]
done
clear
B)
\[\frac{3A}{4}\]
done
clear
C)
4A
done
clear
D)
3A
done
clear
View Answer play_arrow
question_answer 3) A galvanometer of resistance 100\[\Omega \] gives full scale deflection for 20 mV. Find the resistance to be attached, so that it gives full scale deflection of 5 V:
A)
\[24.9\times {{10}^{3}}\Omega inseries\]
done
clear
B)
\[24.9\times {{10}^{3}}\Omega \,\,in\,\,parallel\]
done
clear
C)
\[49.3\times {{10}^{3}}\Omega \,\,in\,\,series\]
done
clear
D)
\[49.3\times {{10}^{3}}\Omega \,\,in\,\,parallel\]
done
clear
View Answer play_arrow
question_answer 4) A convex lens of 40 cm focal length is combined with a concave lens of focal length 25 cm. The power of combination is:
A)
-1.5 D
done
clear
B)
-6.5 D
done
clear
C)
+6.6 D
done
clear
D)
+6.5 D
done
clear
View Answer play_arrow
question_answer 5) A proton and a-particle are having same kinetic energy. Find the ratio of their linear momentum:
A)
\[\sqrt{2}:2\]
done
clear
B)
1: 4
done
clear
C)
2 : 1
done
clear
D)
1 : 2
done
clear
View Answer play_arrow
question_answer 6) A nuclear reactor delivers a power of 10 W. Find fuel consumed by the reactor per hour, if its efficiency is 20%: (Given, \[c=3\times {{10}^{8}}\,m/s\])
A)
\[2\times {{10}^{-6}}g/hr\]
done
clear
B)
\[9\times {{10}^{12}}g/hr\]
done
clear
C)
\[8\times {{10}^{-9}}g/hr\]
done
clear
D)
\[2\times {{10}^{-9}}g/hr\]
done
clear
View Answer play_arrow
question_answer 7) There are three wavelengths \[{{10}^{-8}}m,\,\,{{10}^{-2}}m,\,\,{{10}^{8}}\,m\]. Their respective names are:
A)
visible rays, y-rays, ultraviolet rays
done
clear
B)
ultraviolet, microwaves, radiowaves
done
clear
C)
X-rays, visible rays, radiowaves
done
clear
D)
radiowaves, X-rays, microwaves
done
clear
View Answer play_arrow
question_answer 8) Energy of an electron in the second orbit of hydrogen atom is E and the energy of electron in 3rd orbit of He will be:
A)
\[{{E}_{3}}=\frac{16E}{3}\]
done
clear
B)
\[{{E}_{3}}=\frac{16E}{9}\]
done
clear
C)
\[{{E}_{3}}=\frac{4E}{9}\]
done
clear
D)
\[{{E}_{3}}=\frac{4E}{3}\]
done
clear
View Answer play_arrow
question_answer 9) A prism is filled with liquid of refractive index of \[\sqrt{2}\] If angle of prism is \[60{}^\circ \], find angle of minimum deviation
A)
\[75{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[45{}^\circ \]
done
clear
D)
\[30{}^\circ \]
done
clear
View Answer play_arrow
question_answer 10) A disc having mass M and radius R is rotating with angular velocity \[\omega \], another disc of mass 2M and radius R/2 is placed coaxially on the first disc gently. The angular velocity of system will now be:
A)
\[\frac{4\omega }{5}\]
done
clear
B)
\[\frac{2\omega }{5}\]
done
clear
C)
\[\frac{3\omega }{2}\]
done
clear
D)
\[\frac{2\omega }{3}\]
done
clear
View Answer play_arrow
question_answer 11) If a source is moving away from a stationary observer with velocity of light. The frequency observed will be:
A)
one-third
done
clear
B)
doubled
done
clear
C)
halved
done
clear
D)
unchanged
done
clear
View Answer play_arrow
question_answer 12) A coin is placed on a turn table rotating with \[\omega \], slip at a distance of 4 cm from centre. The distance at which it will slip when co is double, is:
A)
8 cm
done
clear
B)
2cm
done
clear
C)
6 cm
done
clear
D)
1 cm
done
clear
View Answer play_arrow
question_answer 13) If temperature is changed from \[27{}^\circ C\] to \[327{}^\circ C\]. Find ratio of K.E. of molecules at two temperatures:
A)
3 : 2
done
clear
B)
2 : 3
done
clear
C)
1 : 2
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 14) If range and height of a projectile are equal, then angle of projection with the horizontal is:
A)
\[60{}^\circ \]
done
clear
B)
\[{{\tan }^{-1}}\,(4)\]
done
clear
C)
\[30{}^\circ \]
done
clear
D)
\[45{}^\circ \]
done
clear
View Answer play_arrow
question_answer 15) A particle under uniform circular motion is having angular momentum L. If K.E. of the particle is halved and the frequency is doubled, find the new angular momentum:
A)
\[\frac{L}{2}\]
done
clear
B)
\[\frac{L}{4}\]
done
clear
C)
4L
done
clear
D)
2L
done
clear
View Answer play_arrow
question_answer 16) Two equal and opposite charges of \[2\times {{10}^{-10}}C\]are placed at a distance of 1 cm forming a dipole and are placed in an electric field of \[2\times {{10}^{5}}\]N/C. The maximum torque on dipole is:
A)
\[2\sqrt{2}\times {{10}^{-6}}Nm\]
done
clear
B)
\[8\times {{10}^{8}}Nm\]
done
clear
C)
\[4\times {{10}^{-9}}Nm\]
done
clear
D)
\[4\times {{10}^{-7}}Nm\]
done
clear
View Answer play_arrow
question_answer 17) If position vector of mass 1 kg and 3 kg are \[\hat{i}+\hat{j}+\hat{k}\]and \[-\hat{i}-\hat{j}-\hat{k}\]respectively. The final position vector of centre of mass is:
A)
\[-\frac{3}{4}(\hat{i}-\hat{j}-\hat{k})\]
done
clear
B)
\[-\frac{1}{4}(\hat{i}-\hat{j}-\hat{k})\]
done
clear
C)
\[-\frac{1}{2}(\hat{i}-\hat{j}-\hat{k})\]
done
clear
D)
\[-(\hat{i}-\hat{j}-\hat{k})\]
done
clear
View Answer play_arrow
question_answer 18) A particle of mass 4 kg is acted upon by steady force of 4 N. Distance travelled by the particle in 4 sec is:
A)
16 m
done
clear
B)
2 m
done
clear
C)
8 m
done
clear
D)
4 m
done
clear
View Answer play_arrow
question_answer 19) If P.D. across a capacitor is changed from 15 V to 30 V, work done is W. What will be the work done when P.D. is changed from 30 V to 60 V?
A)
W
done
clear
B)
4W
done
clear
C)
3W
done
clear
D)
2W
done
clear
View Answer play_arrow
question_answer 20) If an object is placed at 10 cm in front of a concave mirror of focal length 15 cm. The magnification of image is:
A)
-1.5
done
clear
B)
1.5
done
clear
C)
-3
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 21) A certain electric motor wire that carry a current of 4 amp is perpendicular to a magnetic field of 0.8 T. What is the force on each cm of the wire?
A)
0.08 N
done
clear
B)
0.06 N
done
clear
C)
0.04 N
done
clear
D)
0.02 N
done
clear
View Answer play_arrow
question_answer 22) If the momentum of a particle is doubled, then its de-Broglie wavelength will:
A)
remain unchanged
done
clear
B)
become four times
done
clear
C)
become two times
done
clear
D)
become half
done
clear
View Answer play_arrow
question_answer 23) A hot electric iron has a resistance of 80\[\Omega \] and is used on a 200 V source. The electrical energy spent in using it for two hours, will be:
A)
8000 Wh
done
clear
B)
2000 Wh
done
clear
C)
1000 Wh
done
clear
D)
800 Wh
done
clear
View Answer play_arrow
question_answer 24) The phase difference between two points separated by 1 m in a wave of frequency 120 Hz is 90°. The wave velocity will be:
A)
720 m/s
done
clear
B)
480 m/s
done
clear
C)
240 m/s
done
clear
D)
180 m/s
done
clear
View Answer play_arrow
question_answer 25) Which of following is responsible for the flow of current in a conductor?
A)
Protons and holes
done
clear
B)
Free electrons
done
clear
C)
Positive ions
done
clear
D)
Protons
done
clear
View Answer play_arrow
question_answer 26) A particle is having potential energy 1/3 of the maximum value at a distance of 4 cm from mean position. Amplitude of motion is:
A)
\[\frac{4}{\sqrt{3}}\]
done
clear
B)
\[\frac{6}{\sqrt{2}}\]
done
clear
C)
\[\frac{2}{\sqrt{6}}\]
done
clear
D)
\[2\sqrt{6}\]
done
clear
View Answer play_arrow
question_answer 27) Light is refracted \[\mu \]= 3/2 to water \[\mu \] = 4/3. For total internal reflection sin i will be equal to:
A)
\[{{\sin }^{-1}}\left( \frac{9}{8} \right)\]
done
clear
B)
\[{{45}^{0}}\]
done
clear
C)
\[{{60}^{0}}\]
done
clear
D)
\[{{\sin }^{-1}}\left( \frac{8}{9} \right)\]
done
clear
View Answer play_arrow
question_answer 28) If a particle is rotating in a circle, what will happen?
A)
No force is acting on particle
done
clear
B)
Velocity of particle is constant
done
clear
C)
Particle has no acceleration
done
clear
D)
No work is done
done
clear
View Answer play_arrow
question_answer 29) If a dielectric plate of thickness t is placed between the plates of a parallel plate capacitor of plate distance d, the capacitance becomes half of the original value. The dielectric constant of the plate will be:
A)
\[\frac{2t}{2d+t}\]
done
clear
B)
\[\frac{2t}{2d-t}\]
done
clear
C)
\[\frac{t}{d+t}\]
done
clear
D)
\[\frac{t}{d-t}\]
done
clear
View Answer play_arrow
question_answer 30) In Youngs double slit experiment the intencity at centre of screen is 1. If one of the slit is closed, the intensity at centre now will be:
A)
\[\frac{I}{2}\]
done
clear
B)
\[I\]
done
clear
C)
\[\frac{I}{4}\]
done
clear
D)
\[\frac{I}{3}\]
done
clear
View Answer play_arrow
question_answer 31)
What will be ratio of speed in first two seconds to the speed in next 4 seconds:
A)
\[\sqrt{2}\]: 1
done
clear
B)
3: 1
done
clear
C)
2 :1
done
clear
D)
1: 2
done
clear
View Answer play_arrow
question_answer 32) If an electron jumps from 1st orbit to 3rd orbit, then it will:
A)
not loose energy
done
clear
B)
not given energy
done
clear
C)
release energy
done
clear
D)
absorb energy
done
clear
View Answer play_arrow
question_answer 33) In a coil of self-inductance 5 henry, the rate of change of current is 2 amp per second. The emf induced in the coil is:
A)
-5V
done
clear
B)
5
done
clear
C)
-10 V
done
clear
D)
10 V
done
clear
View Answer play_arrow
question_answer 34) To find the radius of a circular path of an electron, when subjected to a perpendicular magnetic field is:
A)
\[\frac{m\upsilon }{Be}\]
done
clear
B)
\[\frac{me}{B}\]
done
clear
C)
\[\frac{mE}{B}\]
done
clear
D)
\[\frac{Be}{m\upsilon }\]
done
clear
View Answer play_arrow
question_answer 35) The potential difference between the cathode and the target in a Coolidge tube is 120 kV. What can be the minimum wavelength (in \[\overset{0}{\mathop{A}}\,\]) of the X-rays emitted by this tube?
A)
0.4\[\overset{0}{\mathop{A}}\,\]
done
clear
B)
0.3\[\overset{0}{\mathop{A}}\,\]
done
clear
C)
0.2 \[\overset{0}{\mathop{A}}\,\]
done
clear
D)
0.1\[\overset{0}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 36) Wavelength of a light emitted from second orbit to first orbit in a hydrogen atom is:
A)
\[1.215\,\times {{10}^{-7}}\,m\]
done
clear
B)
\[1.215\,\times {{10}^{-5}}\,m\]
done
clear
C)
\[1.215\,\times {{10}^{-4}}m\]
done
clear
D)
\[1.215\,\times {{10}^{-3}}m\]
done
clear
View Answer play_arrow
question_answer 37) Which of the following has lowest ionization potential?
A)
Sr
done
clear
B)
Sn
done
clear
C)
Sb
done
clear
D)
Xe
done
clear
View Answer play_arrow
question_answer 38) Which of the following is not tetrahedral?
A)
\[SiC{{l}_{4}}\]
done
clear
B)
\[B{{F}_{4}}\]
done
clear
C)
\[N{{H}_{3}}\]
done
clear
D)
\[SeC{{l}_{4}}\]
done
clear
View Answer play_arrow
question_answer 39) Zwitter ion is:
A)
a molecule with positive charge having one atom less
done
clear
B)
a molecule having both negative and positive charge on opposite ends.
done
clear
C)
a small molecule having only negative charge
done
clear
D)
a molecule having only positive charge on one of its atom
done
clear
View Answer play_arrow
question_answer 40) The compound in brown ring test is:
A)
\[[Fe{{({{H}_{2}}O)}_{5}}NO]S{{O}_{4}}\]
done
clear
B)
\[[Fe{{({{H}_{2}}O)}_{4}}NO]S{{O}_{4}}\]
done
clear
C)
\[[Fe({{H}_{2}}O)N{{O}_{2}}]S{{O}_{4}}\]
done
clear
D)
none Of these
done
clear
View Answer play_arrow
question_answer 41) I.U.P.A.C. name of: \[{{H}_{3}}-\underset{H}{\overset{Br}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{Cl}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,=C{{H}_{2}}\] is:
A)
4-bromo 3- chloro pent-1-ene
done
clear
B)
2-bromo 3-chloro pentene
done
clear
C)
4-bromo 2-chloro butene-1
done
clear
D)
4-methyl 4-bromo 3-chloro butene
done
clear
View Answer play_arrow
question_answer 42) When aniline is heated to \[{{440}^{o}}C-{{400}^{o}}C\] with sulphuric acid, it will give:
A)
sulphanilic acid
done
clear
B)
aniline hydrogen sulphate
done
clear
C)
aniline sulphite
done
clear
D)
benzene sulphonic acid
done
clear
View Answer play_arrow
question_answer 43) Which of the following does not show Tyndal effect?
A)
True solution
done
clear
B)
Suspension
done
clear
C)
Emulsion
done
clear
D)
Gel
done
clear
View Answer play_arrow
question_answer 44) \[n=4,l=2\]and \[m=0,\]refer to:
A)
4s
done
clear
B)
4p
done
clear
C)
4d
done
clear
D)
\[4f\]
done
clear
View Answer play_arrow
question_answer 45) Which of the following 0.1 M aqueous solution will have lowest freezing point?
A)
Urea
done
clear
B)
Glucose
done
clear
C)
\[\text{NaCl}\]
done
clear
D)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
View Answer play_arrow
question_answer 46) When chloroform reacts with phenol in the presence of KOH (aq.), the reaction is called:
A)
Wurtz reaction
done
clear
B)
Reimer-Tiemann reaction
done
clear
C)
Friedel-Crafts reaction
done
clear
D)
Perkin reaction
done
clear
View Answer play_arrow
question_answer 47) Which of the following is an ionic hydride?
A)
\[\text{NaH}\]
done
clear
B)
\[\text{HCl}\]
done
clear
C)
\[\text{N}{{\text{H}}_{3}}\]
done
clear
D)
\[\text{Si}{{\text{H}}_{\text{4}}}\]
done
clear
View Answer play_arrow
question_answer 48) The percentage of gold in 20 carat gold is:
A)
95
done
clear
B)
83.33
done
clear
C)
80
done
clear
D)
38.67
done
clear
View Answer play_arrow
question_answer 49) Lucas reagent will give instant turbidity with:
A)
primary alcohol
done
clear
B)
secondary alcohol
done
clear
C)
tertiary alcohol
done
clear
D)
methane
done
clear
View Answer play_arrow
question_answer 50) Which of the following is most stable?
A)
\[\overset{\text{+}}{\mathop{\text{C}}}\,{{\text{H}}_{\text{3}}}\]
done
clear
B)
\[{{\text{R}}_{2}}\overset{+}{\mathop{C}}\,H\]
done
clear
C)
\[{{R}_{3}}\overset{+}{\mathop{C}}\,\]
done
clear
D)
\[R\overset{+}{\mathop{C}}\,{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 51) if solubility product of \[\text{AgCl}\] is \[\text{1}{{\text{0}}^{-10}}\] (mol \[\omega t=143.35\]), then its solubility in gram/litre will be:
A)
\[143.5\times {{10}^{-5}}\]
done
clear
B)
\[143.5\times {{10}^{-2}}\]
done
clear
C)
\[14.35\times {{10}^{-1}}\]
done
clear
D)
\[1.435\times {{10}^{-6}}\]
done
clear
View Answer play_arrow
question_answer 52) The bond order in \[\text{C}_{\text{2}}^{\text{+}}\] is:
A)
1
done
clear
B)
2
done
clear
C)
0.5
done
clear
D)
1.5
done
clear
View Answer play_arrow
question_answer 53) \[E{{_{Zn}^{o}}^{2+}}_{/Zn}=-0.7\text{6}\,\text{V}\]and \[E_{A{{g}^{+}}/Ag}^{o}=+\,0.80\,\text{V}\]What is \[E_{cell}^{o}\]?
A)
\[+\,2.34\,V\]
done
clear
B)
\[+1.56\,V\]
done
clear
C)
\[-1.56\,V\]
done
clear
D)
\[-2.34\,V\]
done
clear
View Answer play_arrow
question_answer 54) Terylene is the polymer of terephthalic acid and:
A)
\[C{{H}_{2}}OHC{{H}_{2}}OH\]
done
clear
B)
done
clear
C)
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 55) Pressure in increased with temperature for constant volume and fixed mass of a gas due to:
A)
increase in intermolecular attraction
done
clear
B)
decrease in the mean free path
done
clear
C)
increase in the collision between molecule
done
clear
D)
increase of K.E of molecule
done
clear
View Answer play_arrow
question_answer 56) Which of the following is not metalloid?
A)
Si
done
clear
B)
Se
done
clear
C)
As
done
clear
D)
Te
done
clear
View Answer play_arrow
question_answer 57) Which of the following compound is formed by Na on fusion with an organic compound having nitrogen as an extra element:
A)
\[NaN\]
done
clear
B)
\[NaCN\]
done
clear
C)
\[~NaNO\]
done
clear
D)
\[NaC\]
done
clear
View Answer play_arrow
question_answer 58) The formula of cryolite is:
A)
\[N{{a}_{3}}Al{{F}_{6}}\]
done
clear
B)
\[CaC{{l}_{2}}.A{{l}_{2}}{{(S{{O}_{4}})}_{3}}\]
done
clear
C)
\[F{{e}_{4}}[Fe{{(CN)}_{6}}]\]
done
clear
D)
\[A{{l}_{2}}{{O}_{3}}.2{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 59)
In the reaction,
A)
benzene diazonium chloride and azodye
done
clear
B)
nitrobenzene and o-nitrophenol
done
clear
C)
benzene and salicylic acid
done
clear
D)
chlorobenzene and naphthalene
done
clear
View Answer play_arrow
question_answer 60) The weight of one molecule of the compound \[{{\text{C}}_{\text{60}}}{{\text{H}}_{\text{122}}}\]is:
A)
\[1.4\times {{10}^{-21}}g\]
done
clear
B)
\[5.025\times {{10}^{-23}}g\]
done
clear
C)
\[1.3\times {{10}^{-19}}g\]
done
clear
D)
\[2.30\times {{10}^{-22}}g\]
done
clear
View Answer play_arrow
question_answer 61) An ideal gas is heated \[\text{27}{{\,}^{\text{o}}}\text{C}\]to \[\text{627}{{\,}^{o}}\text{C}\]at constant pressure. If initial volume was \[\text{4}\,{{\text{m}}^{\text{2}}}\text{,}\] then the final volume of the gas will be:
A)
\[\text{2}\,{{\text{m}}^{3}}\]
done
clear
B)
\[8\,{{\text{m}}^{3}}\]
done
clear
C)
\[12\,{{m}^{3}}\]
done
clear
D)
\[\text{10}\,{{\text{m}}^{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 62) In the nuclear reaction \[{{\,}_{6}}{{C}^{13}}+{{\,}_{1}}{{H}^{1}}\xrightarrow{{}}{{\,}_{7}}{{N}^{14}}+A,A\]
A)
\[\alpha -\]particle
done
clear
B)
\[\gamma -\]particle
done
clear
C)
\[\beta -\] particle
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 63) pH of \[\text{1}{{\text{0}}^{-8}}\,\text{MHCl}\]is:
A)
6.96
done
clear
B)
8
done
clear
C)
6
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 64) The half life is 5 min. How much % of radioactive compound remains after 20 minutes?
A)
6.25%
done
clear
B)
93.75%
done
clear
C)
12.5%
done
clear
D)
87.5%
done
clear
View Answer play_arrow
question_answer 65) At S.T.P. \[\,\text{10}\,\,\text{g}\,\,\text{CaC}{{\text{O}}_{\text{3}}}\]on decomposition will librates \[\text{C}{{\text{O}}_{2}}:\]
A)
22.4 litre
done
clear
B)
10 litre
done
clear
C)
2.24 litre
done
clear
D)
15 litre
done
clear
View Answer play_arrow
question_answer 66) The density of \[\text{HCl}\] is 1.21 g/mL. Its normality is:
A)
33.15 N
done
clear
B)
18.3 N
done
clear
C)
198 N
done
clear
D)
35 N
done
clear
View Answer play_arrow
question_answer 67) DDT is used as:
A)
insecticide
done
clear
B)
antibiotic
done
clear
C)
antiseptic
done
clear
D)
herbicide
done
clear
View Answer play_arrow
question_answer 68) \[{{H}_{3}}C-C\equiv C-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{C}}\,}}\,=C{{H}_{2}}\] has how many \[\sigma \] and \[\pi \] bonds?
A)
13, 3
done
clear
B)
10, 3
done
clear
C)
15, 4
done
clear
D)
12, 4
done
clear
View Answer play_arrow
question_answer 69) Hybridization in \[\text{PC}{{\text{l}}_{\text{3}}}\]is:
A)
\[\text{s}{{\text{p}}^{3}}\]
done
clear
B)
\[s{{p}^{2}}\]
done
clear
C)
\[s{{p}^{3}}d\]
done
clear
D)
\[s{{p}^{1}}\]
done
clear
View Answer play_arrow
question_answer 70) Oxidation number of aluminium in \[\text{N}{{\text{a}}_{\text{3}}}\text{Al}{{\text{F}}_{\text{6}}}\] is:
A)
+ 3
done
clear
B)
+ 2
done
clear
C)
- 2
done
clear
D)
+ 1
done
clear
View Answer play_arrow
question_answer 71) The E.A.N. of Ni in \[\text{Ni(CO}{{\text{)}}_{\text{4}}}\] is:
A)
36
done
clear
B)
34
done
clear
C)
32
done
clear
D)
30
done
clear
View Answer play_arrow
question_answer 72) Which has the maximum percentage of chlorine?
A)
\[{{C}_{6}}{{H}_{6}}C{{l}_{6}}\]
done
clear
B)
\[CHC{{l}_{3}}\]
done
clear
C)
\[{{C}_{6}}{{H}_{5}}Cl\]
done
clear
D)
\[C{{H}_{3}}Cl\]
done
clear
View Answer play_arrow
question_answer 73) Electronic configuration of element with (Z = 24) is :
A)
\[1{{s}^{2}},2{{s}^{2}}2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}3{{d}^{5}},4{{s}^{1}}\]
done
clear
B)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{4}}4{{s}^{2}}\]
done
clear
C)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{3}}\]
done
clear
D)
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{d}^{5}}3{{s}^{2}}3{{p}^{6}}\]
done
clear
View Answer play_arrow
question_answer 74) \[\text{CuS}{{\text{O}}_{\text{4}}}\]reacts with \[\text{N}{{\text{H}}_{\text{4}}}\text{OH}\]to give deep blue complex of:
A)
cupra ammonium hydroxide
done
clear
B)
cupra ammonium sulphate
done
clear
C)
Both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 75) The product of pressure and volume (PV) has a unit of:
A)
force
done
clear
B)
energy
done
clear
C)
entropy
done
clear
D)
impulse
done
clear
View Answer play_arrow
question_answer 76) 2, 4-D is an effective:
A)
fungicide
done
clear
B)
weedicide
done
clear
C)
rodenticide
done
clear
D)
wormicide
done
clear
View Answer play_arrow
question_answer 77) The term meiosis was given by:
A)
Johanson
done
clear
B)
Knoll and Ruska
done
clear
C)
Flemming
done
clear
D)
Farmer and Moore
done
clear
View Answer play_arrow
question_answer 78) Puccinia forms uredia and:
A)
pycnia on barberry leaves
done
clear
B)
aecia on wheat leaves
done
clear
C)
telia on wheat leaves
done
clear
D)
aecia on barberry leaves
done
clear
View Answer play_arrow
question_answer 79) Two bacteria found to be very useful in genetic engineering experiments are:
A)
Nitrosomonas and Kliebsiella
done
clear
B)
Escherichia and Agrobacterium
done
clear
C)
Nitrobacter and Azotobacter
done
clear
D)
Rhizobium and Diplococcus
done
clear
View Answer play_arrow
question_answer 80) Parents of blood group O will have offsprings of:
A)
O only
done
clear
B)
A and O
done
clear
C)
O and B
done
clear
D)
O and AB
done
clear
View Answer play_arrow
question_answer 81) The periderm includes:
A)
cork
done
clear
B)
cambium
done
clear
C)
secondary cortex
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 82) Genetic drift operates only in:
A)
island populations
done
clear
B)
smaller populations
done
clear
C)
larger populations
done
clear
D)
Mendelian populations
done
clear
View Answer play_arrow
question_answer 83) Which one of the following statement is correct?
A)
Homo erectus is the ancestor of man
done
clear
B)
Cro-magnon mans fossil has beer found in Ethopia
done
clear
C)
Australopithecus is the real ancestor cc modern man
done
clear
D)
Neanderthal man is the direct ancestor of Homo sapiens
done
clear
View Answer play_arrow
question_answer 84) When a single gene influences more than one trait it is called:
A)
pleiotropy
done
clear
B)
epistasis
done
clear
C)
pseudo-dominance
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 85) The exchanges of gases in the alveoli cc the lungs takes place by:
A)
osmosis
done
clear
B)
simple diffusion
done
clear
C)
passive transport
done
clear
D)
active transport
done
clear
View Answer play_arrow
question_answer 86) Phytochrome becomes active in:
A)
green light
done
clear
B)
blue light
done
clear
C)
red light
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 87) Which of the following is not a vital disease?
A)
Rabies
done
clear
B)
Chicken-pox
done
clear
C)
Leprosy
done
clear
D)
Polio
done
clear
View Answer play_arrow
question_answer 88) Heterocyst occurs in:
A)
Ulothrix
done
clear
B)
Spirogyra
done
clear
C)
Nostoc
done
clear
D)
Chlamydomonas
done
clear
View Answer play_arrow
question_answer 89) Colchicine is an inhibitory chemical, which:
A)
prevents the spindle formation in mitosis
done
clear
B)
prevents the formation of equatorial plane
done
clear
C)
stops the functioning of centriole
done
clear
D)
prevents attaching of centromeres with rays:
done
clear
View Answer play_arrow
question_answer 90) The polygenic genes show:
A)
different genotypes
done
clear
B)
different phenotypes
done
clear
C)
different karyotypes
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 91) Maximum absorption of light occurs in the region of:
A)
400-700 nm
done
clear
B)
700-900 nm
done
clear
C)
1000-1200 nm
done
clear
D)
1500-2000 nm
done
clear
View Answer play_arrow
question_answer 92) Non symbiotic nitrogen fixation is done by:
A)
Frankia
done
clear
B)
Anabaena
done
clear
C)
Azotobacter
done
clear
D)
Rhizobiwn
done
clear
View Answer play_arrow
question_answer 93) Digestion of fat is mainly facilitated by:
A)
bile juice
done
clear
B)
pancreatic juice
done
clear
C)
amylase
done
clear
D)
gastric juice
done
clear
View Answer play_arrow
question_answer 94) Lactose is composed of:
A)
glucose + fructose
done
clear
B)
glucose + glucose
done
clear
C)
glucose + galactose
done
clear
D)
fructose + galactose
done
clear
View Answer play_arrow
question_answer 95) H.J. Muller had received Nobel prize for:
A)
discovering the linkage of genes
done
clear
B)
discovering the induced mutations by X-rays
done
clear
C)
his studies on Drosophila for genetic study
done
clear
D)
proving that the DNA is a genetic material
done
clear
View Answer play_arrow
question_answer 96) Acrosome of sperm head is formed by:
A)
golgi body
done
clear
B)
nucleus
done
clear
C)
endoplasmic reticulum
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 97) Which hormones stimulates the secretion of milk from female?
A)
LH
done
clear
B)
Prolactin
done
clear
C)
Oxytocin
done
clear
D)
Progesterone
done
clear
View Answer play_arrow
question_answer 98) Coelom is found between the cavity of:
A)
ectoderm and endoderm
done
clear
B)
mesoderm and ectoderm
done
clear
C)
endoderm and ectoderm
done
clear
D)
mesoderm and endoderm
done
clear
View Answer play_arrow
question_answer 99) Same species found in different geographical areas are:
A)
sympatric
done
clear
B)
allopatric
done
clear
C)
siblings
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 100) AIDS disease was first reported in:
A)
Russia
done
clear
B)
Germany
done
clear
C)
USA
done
clear
D)
France
done
clear
View Answer play_arrow
question_answer 101) In \[{{C}_{4}}\] plants, \[C{{O}_{2}}\] fixation is done by:
A)
mesophyll cells
done
clear
B)
guard cells
done
clear
C)
sclerenchyma
done
clear
D)
chlorenchyma and hypodermis
done
clear
View Answer play_arrow
question_answer 102) Electron microscope was invented by
A)
Watson and Crick
done
clear
B)
Knoll and Ruska
done
clear
C)
H.J. Muller
done
clear
D)
Aristotle
done
clear
View Answer play_arrow
question_answer 103) The black rust of wheat is a fungal disease caused by:
A)
Melampsora lini
done
clear
B)
Claviceps purpurea
done
clear
C)
Albugo Candida
done
clear
D)
Puccinia graminis tritici
done
clear
View Answer play_arrow
question_answer 104) Curvature of coleoptile tip in an experiment was due to:
A)
IAA
done
clear
B)
cytokinin
done
clear
C)
ethylene
done
clear
D)
ABA
done
clear
View Answer play_arrow
question_answer 105) The twinning of tendrils around a support is a good example of:
A)
nastic movement
done
clear
B)
thigmotropism
done
clear
C)
phototropism
done
clear
D)
chemotropism
done
clear
View Answer play_arrow
question_answer 106) When a cell is fully turgid which of the following will be zero?
A)
Wall pressure
done
clear
B)
Osmotic pressure
done
clear
C)
Turgor pressure
done
clear
D)
Water potential
done
clear
View Answer play_arrow
question_answer 107) DNA synthesis can be specifically measured by estimating the incorporation of radio-labeled:
A)
uracil
done
clear
B)
adenine
done
clear
C)
thymine
done
clear
D)
deoxyribose sugar
done
clear
View Answer play_arrow
question_answer 108) How many mitotic divisions are needed for a single cell to make 128 cells?
A)
7
done
clear
B)
14
done
clear
C)
28
done
clear
D)
32
done
clear
View Answer play_arrow
question_answer 109) Co-factor (prosthetic group) is a part of holoenzyme. It is:
A)
loosely attached inorganic part
done
clear
B)
accessory non-protein substance attached firmly
done
clear
C)
loosely attached organic part
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 110) Bryophytes can be separated from algae, because they:
A)
are thalloid forms
done
clear
B)
have no conducting tissue
done
clear
C)
possess archegonia
done
clear
D)
contain chloroplast
done
clear
View Answer play_arrow
question_answer 111) The core metal of chlorophyll is:
A)
Fe
done
clear
B)
Mg
done
clear
C)
Ni
done
clear
D)
Cu
done
clear
View Answer play_arrow
question_answer 112) Inferior ovary is found in:
A)
Labiatae
done
clear
B)
Liliaceae
done
clear
C)
Solanaceae
done
clear
D)
Compositae
done
clear
View Answer play_arrow
question_answer 113) The end product of fermentation are:
A)
\[C{{O}_{2}}\] and \[{{O}_{2}}\]
done
clear
B)
\[C{{O}_{2}}\] and \[{{C}_{2}}{{H}_{5}}OH\]
done
clear
C)
\[{{O}_{2}}\] and \[{{C}_{2}}{{H}_{5}}OH\]
done
clear
D)
\[C{{O}_{2}}\] and \[C{{H}_{3}}CHO\]
done
clear
View Answer play_arrow
question_answer 114) The desmosomes are concerned with:
A)
cytolysis
done
clear
B)
cellular excretion
done
clear
C)
cell division
done
clear
D)
cell adherence
done
clear
View Answer play_arrow
question_answer 115) The lymph serves to:
A)
transport \[C{{O}_{2}}\] to the lungs
done
clear
B)
transport \[{{O}_{2}}\] to the brain
done
clear
C)
return the interstitial fluid to the b.
done
clear
D)
return the WBCs and RBCs to - lymph nodes
done
clear
View Answer play_arrow
question_answer 116) Which of the following cycle, found sugarcane?
A)
Calvin cycle
done
clear
B)
Hatch and Slack cycle
done
clear
C)
CAM-cycle
done
clear
D)
EMP-pathway
done
clear
View Answer play_arrow
question_answer 117) Fallopian tube is lined by:
A)
pseudostratified epithelium
done
clear
B)
ciliated epithelium
done
clear
C)
cuboidal epithelium
done
clear
D)
squamous epithelium
done
clear
View Answer play_arrow
question_answer 118) Tallest tree belongs to:
A)
gymnosperms
done
clear
B)
monocots
done
clear
C)
dicots
done
clear
D)
pteridophytes
done
clear
View Answer play_arrow
question_answer 119) Identify the correct match between tiger reserve and its state:
A)
Bandipur - Tamil Nadu
done
clear
B)
Palau - Orissa
done
clear
C)
Manas - Assam
done
clear
D)
Corbett - Madhya Pradesh
done
clear
View Answer play_arrow
question_answer 120) In abiotic community, the primar consumers are:
A)
carnivores
done
clear
B)
omnivores
done
clear
C)
detrivores
done
clear
D)
herbivores
done
clear
View Answer play_arrow
question_answer 121) Barr body in mammals represents:
A)
all the heterochromatin in female cells
done
clear
B)
one of the two X-chromosomes in somatic cells of females
done
clear
C)
all the heterochromatin in male an; female cells
done
clear
D)
the Y-chromosome in somatic cell; male
done
clear
View Answer play_arrow
question_answer 122) Pinus differ from mango in:
A)
covered ovules
done
clear
B)
uncovered ovules
done
clear
C)
diploid endosperm
done
clear
D)
colour of leaves
done
clear
View Answer play_arrow
question_answer 123) When a viral DNA integrates with a h - DNA then the process is referred as:
A)
lysis
done
clear
B)
prophage
done
clear
C)
lysogeny
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 124) The blood cancer is known as:
A)
leukaemia
done
clear
B)
thrombosis
done
clear
C)
haemolysis
done
clear
D)
haemophilia
done
clear
View Answer play_arrow
question_answer 125) How many chromosomes are present in ovum of man?
A)
22 + X
done
clear
B)
22 + Y
done
clear
C)
44 + X
done
clear
D)
44 + Y
done
clear
View Answer play_arrow
question_answer 126) Life was originated in:
A)
water
done
clear
B)
air
done
clear
C)
fire
done
clear
D)
soil
done
clear
View Answer play_arrow
question_answer 127) Convergent evolution is shown by:
A)
rabbit and dog
done
clear
B)
fish and whale
done
clear
C)
bacteria and Amoeba
done
clear
D)
starfish and jelly fish
done
clear
View Answer play_arrow
question_answer 128) The life span of human WBC is approximately:
A)
less than 10 days
done
clear
B)
between 20 to 30 days
done
clear
C)
between 2 to 3 months
done
clear
D)
more than 4 months
done
clear
View Answer play_arrow
question_answer 129) T. Schwann and M-Schleiden were:
A)
Dutch biologists
done
clear
B)
Austrian biologists
done
clear
C)
German biologists
done
clear
D)
English biologists
done
clear
View Answer play_arrow
question_answer 130) Lvsosomes are the store house of:
A)
hydrolytic enzymes
done
clear
B)
proteins
done
clear
C)
sugar
done
clear
D)
ATP
done
clear
View Answer play_arrow
question_answer 131) Which of the following is responsible for the mechanical support, protein synthesis and enzyme transport?
A)
Endoplasmic reticulum
done
clear
B)
Mitochondria
done
clear
C)
Cell membrane
done
clear
D)
Dictyosome
done
clear
View Answer play_arrow
question_answer 132) The kingdoms-Monera, Protista, Fungi, Plantae and Animalia are distinguished on the basis of:
A)
type of nutrition
done
clear
B)
type of cell
done
clear
C)
type of reproduction
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 133) The protists have:
A)
membrane bound nucleoproteins lying embedded is the cytoplasm
done
clear
B)
gene containing nucleoproteins condensed together in loss mass
done
clear
C)
nucleoproteins is direct contact with the rest of the cell substance
done
clear
D)
only free nucleic acid aggregates
done
clear
View Answer play_arrow
question_answer 134) Which of the following is obtained from algae?
A)
Carragenin
done
clear
B)
Chocolate
done
clear
C)
Butter
done
clear
D)
Wax
done
clear
View Answer play_arrow
question_answer 135) Which of the following plant kingdom is called amphibians?
A)
Thallophyta
done
clear
B)
Pteridophyta
done
clear
C)
Tracheophyta
done
clear
D)
Bryophyta
done
clear
View Answer play_arrow
question_answer 136) Enzymes are made up of:
A)
proteins
done
clear
B)
fats
done
clear
C)
carbohydrates
done
clear
D)
lipids
done
clear
View Answer play_arrow
question_answer 137) Coconut milk represents
A)
mesocarp
done
clear
B)
endocarp
done
clear
C)
epicarp
done
clear
D)
endosperm
done
clear
View Answer play_arrow
question_answer 138) Which of the following is grouped under phanerogams?
A)
Pteridophyta
done
clear
B)
Angiosperms
done
clear
C)
Gymnosperms
done
clear
D)
Both b and c
done
clear
View Answer play_arrow
question_answer 139) lpomoea batata belongs to family:
A)
Ranunculaceae
done
clear
B)
Rutaceae
done
clear
C)
Gramineae
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 140) Anticodon of f-RNA binds with:
A)
nucleic bases of m-RNA
done
clear
B)
nucleic bases of r-RNA
done
clear
C)
codons of f-RNA
done
clear
D)
amino acids
done
clear
View Answer play_arrow
question_answer 141) Which of the following causes white rust of crucifers?
A)
Aspergillus
done
clear
B)
Puccinia
done
clear
C)
Mucor
done
clear
D)
Albugo Candida
done
clear
View Answer play_arrow
question_answer 142) Treeless terrestrial biome of cold climates is:
A)
plankton
done
clear
B)
tundra
done
clear
C)
savanna
done
clear
D)
taiga
done
clear
View Answer play_arrow
question_answer 143) Which of the following cell organelle is associated with protein synthesis?
A)
Ribosome
done
clear
B)
Microsome
done
clear
C)
Lysosome
done
clear
D)
Glyoxysome
done
clear
View Answer play_arrow
question_answer 144) The pyramid of energy is always:
A)
inverted
done
clear
B)
horizontal
done
clear
C)
both a and b
done
clear
D)
upright
done
clear
View Answer play_arrow
question_answer 145) Loss of relative biological equilibrium is due to:
A)
low temperature
done
clear
B)
high temperature
done
clear
C)
radiation
done
clear
D)
pollution
done
clear
View Answer play_arrow
question_answer 146) Apical dominance is due to:
A)
methylene
done
clear
B)
auxin
done
clear
C)
Abscissic acid
done
clear
D)
ethylene
done
clear
View Answer play_arrow
question_answer 147) In which of the following form, sugar is transported within the body of plant?
A)
Maltose
done
clear
B)
Glucose
done
clear
C)
Lactose
done
clear
D)
Sucrose
done
clear
View Answer play_arrow
question_answer 148) The process of transfer of DNA from bacterium to another through virus called:
A)
transformation
done
clear
B)
transduction
done
clear
C)
conjugation
done
clear
D)
termination
done
clear
View Answer play_arrow
question_answer 149) Oxygen is released from:
A)
cytoplasm
done
clear
B)
golgi body
done
clear
C)
chloroplast
done
clear
D)
mitochondria
done
clear
View Answer play_arrow
question_answer 150) The first reaction in photorespiration
A)
phosphorylation
done
clear
B)
decarboxylation
done
clear
C)
oxygenation
done
clear
D)
carboxylation
done
clear
View Answer play_arrow
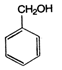
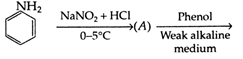 product (A) and (B) are
product (A) and (B) are
