question_answer 1) The light of wavelength 4000 \[\overset{0}{\mathop{A}}\,\] falls on a photosensitive substance whose work function is 2 eV. Its stopping potential is:
A)
1.1 V
done
clear
B)
1.8 V
done
clear
C)
1.26 V
done
clear
D)
0.8 V
done
clear
View Answer play_arrow
question_answer 2) A bullet is fired from gun. The force on the bullet is given by \[F=600\,-2\times {{10}^{5}}\,t,\] where F is in neutron and t in second. The force on the bullet becomes zero as soon as it leaves the barrel. What is the average impulse imparted to the bullet?
A)
1.8 N
done
clear
B)
zero
done
clear
C)
9 N
done
clear
D)
0.9N
done
clear
View Answer play_arrow
question_answer 3) The focal length of a biconvex lens of radii of each surfaces 50 cm and refractive index 1.5, is:
A)
40.4 cm
done
clear
B)
75 cm
done
clear
C)
50 cm
done
clear
D)
80 cm
done
clear
View Answer play_arrow
question_answer 4) If full scale deflection is obtained in a galvanometer of resistance 99 ohm by passing a current of \[{{10}^{-4}}\,A,\] the value of shunt which is connected in parallel measured from it is:
A)
0.0909 \[\Omega \]
done
clear
B)
1.0099\[\Omega \]
done
clear
C)
0.9909 \[\Omega \]
done
clear
D)
0.0099\[\Omega \]
done
clear
View Answer play_arrow
question_answer 5) If in a fission process, the mass defect is 1%, then energy released from the fission of 1 kg substance is:
A)
\[9.0\times {{10}^{13}}J\]
done
clear
B)
\[7.0\,\times {{10}^{13}}J\]
done
clear
C)
\[4.0\times {{10}^{13}}J\]
done
clear
D)
\[5.3\times {{10}^{13}}J\]
done
clear
View Answer play_arrow
question_answer 6) In an electromagnetic wave, the electrical magnetic field are:
A)
mutually parallel
done
clear
B)
mutually perpendicular
done
clear
C)
inclined at an acute angle with each other
done
clear
D)
inclined at an obtuse angle with each other
done
clear
View Answer play_arrow
question_answer 7) A proton and an \[\alpha \]-particle follow the same circular path in a transverse magnetic field. Their kinetic energies are in the ratio:
A)
1 : 4
done
clear
B)
4 : 1
done
clear
C)
1 : 2
done
clear
D)
1 : 1
done
clear
View Answer play_arrow
question_answer 8) The angle of prism is \[60{}^\circ \] and angle of deviation is \[30{}^\circ \]. In the position of minimum deviation, the angle of incidence i and the angle of emergence e are:
A)
\[i=45{}^\circ ,\text{ }e=50{}^\circ \]
done
clear
B)
\[i=30{}^\circ ,\text{ }e=45{}^\circ \]
done
clear
C)
\[i=45{}^\circ ,\text{ }e=45{}^\circ \]
done
clear
D)
\[i=30{}^\circ ,\text{ }e=30{}^\circ \]
done
clear
View Answer play_arrow
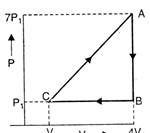
question_answer 9)
In the cyclic process shown in figure, the work done by the gas in one cycle is:
A)
\[28\,{{P}_{1}}{{V}_{1}}\]
done
clear
B)
\[14\,{{P}_{1}}{{V}_{1}}\]
done
clear
C)
\[18\,{{P}_{1}}{{V}_{1}}\]
done
clear
D)
\[9\,{{P}_{1}}{{V}_{1}}\]
done
clear
View Answer play_arrow
question_answer 10) What is the de-Broglie wavelength of the\[\alpha \]-particle accelerated through a potential difference of V volt? (mass of \[\alpha \] particle \[=6.6465\,\times {{10}^{-27}}\,kg\])
A)
\[\frac{0.287}{\sqrt{V}}\overset{0}{\mathop{A}}\,\]
done
clear
B)
\[\frac{12.27}{\sqrt{V}}\overset{0}{\mathop{A}}\,\]
done
clear
C)
\[\frac{0.101}{\sqrt{V}}\overset{0}{\mathop{A}}\,\]
done
clear
D)
\[\frac{0.202}{\sqrt{V}}\overset{0}{\mathop{A}}\,\]
done
clear
View Answer play_arrow
question_answer 11) If \[{{\upsilon }_{rms}}\]of molecules of a diatomic gas is1930 m/s at room temperature \[\left( 27{}^\circ C \right)\], then the gas will be:
A)
\[{{H}_{2}}\]
done
clear
B)
\[{{F}_{2}}\]
done
clear
C)
\[{{O}_{2}}\]
done
clear
D)
\[C{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 12) Two waves are represented by \[{{y}_{1}}=a\sin \left( \omega t+\frac{\pi }{6} \right)\]and\[{{y}_{2}}=a\cos \omega t\] What will be their resultant amplitude?
A)
a
done
clear
B)
\[\sqrt{2}a\]
done
clear
C)
\[\sqrt{3}a\]
done
clear
D)
2a
done
clear
View Answer play_arrow
question_answer 13) A body of mass 4m at rest explodes into three pieces. Two of the pieces each of mass m move with a speed v each in mutually perpendicular directions. The total kinetic energy released is:
A)
\[\frac{1}{2}m{{v}^{2}}\]
done
clear
B)
\[m{{\upsilon }^{2}}\]
done
clear
C)
\[\frac{3}{2}m{{\upsilon }^{2}}\]
done
clear
D)
\[\frac{5}{2}m{{\upsilon }^{2}}\]
done
clear
View Answer play_arrow
question_answer 14) An aeroplane flying horizontally with a speed of \[360\,km{{h}^{-1}}\] releases a bomb at a height of 490 m from the ground. If \[g=9.8\,m{{s}^{-2}}\]. It will strike the ground at:
A)
10 km
done
clear
B)
100 km
done
clear
C)
1 km
done
clear
D)
10 km
done
clear
View Answer play_arrow
question_answer 15) A circular disc of mass m and radius r is rolling forward in horizontal table with a velocity \[\upsilon \]. Its total kinetic energy is:
A)
(a )\[m{{\upsilon }^{2}}\]
done
clear
B)
\[\frac{3}{4}m{{\upsilon }^{2}}\]
done
clear
C)
\[\frac{1}{4}m{{\upsilon }^{2}}\]
done
clear
D)
\[\frac{1}{2}m{{\upsilon }^{2}}\]
done
clear
View Answer play_arrow
question_answer 16) A charged particle of mass m and charge q initially at rest is released in an electric field of magnitude E. Its kinetic energy after time t will be:
A)
\[\frac{2{{E}^{2}}{{t}^{2}}}{mq}\]
done
clear
B)
\[\frac{{{E}^{2}}{{q}^{2}}{{t}^{2}}}{2m}\]
done
clear
C)
\[\frac{E{{q}^{2}}m}{2{{t}^{2}}}\]
done
clear
D)
\[\frac{Eqm}{2t}\]
done
clear
View Answer play_arrow
question_answer 17) Two equal vectors have a resultant equal to either of the two. The angle between them is:
A)
\[90{}^\circ \]
done
clear
B)
\[60{}^\circ \]
done
clear
C)
\[120{}^\circ \]
done
clear
D)
\[0{}^\circ \]
done
clear
View Answer play_arrow
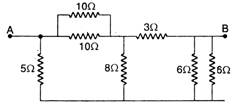
question_answer 18)
Seven resistances are connected as shown in the figure. The equivalent resistance between A and B is:
A)
3\[\Omega \]
done
clear
B)
4\[\Omega \]
done
clear
C)
4.5 \[\Omega \]
done
clear
D)
5\[\Omega \]
done
clear
View Answer play_arrow
question_answer 19) If \[{{m}_{1}}\] and \[{{m}_{2}}\] be the linear magnification of the object in the conjugate positions of a convex lens, and if d be the distance between the conjugate positions, then the focal length of the lens is given by:
A)
\[f=\frac{d}{{{m}_{1}}-{{m}_{2}}}\]
done
clear
B)
\[f=\frac{d}{{{m}_{1}}+{{m}_{2}}}\]
done
clear
C)
\[f=\frac{{{m}_{1}}-{{m}_{2}}}{d}\]
done
clear
D)
either (a) and (b)
done
clear
View Answer play_arrow
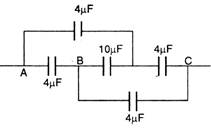
question_answer 20)
Equivalent capacitance of the given combination of five capacitors is:
A)
4 \[\mu F\]
done
clear
B)
10\[\mu F\]
done
clear
C)
8 \[\mu F\]
done
clear
D)
120\[\mu F\]
done
clear
View Answer play_arrow
question_answer 21) Suppose the number of turns in a coil be tripled, the value of magnetic flux linked with it:
A)
remains unchanged
done
clear
B)
becomes 1/3
done
clear
C)
is tripled
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 22) The ratio of minimum to maximum wavelength in Balmer series is:
A)
5 : 9
done
clear
B)
5 : 36
done
clear
C)
1 : 4
done
clear
D)
3 : 4
done
clear
View Answer play_arrow
question_answer 23) When a junction diode is reverse biased, the flow of current across the junction is mainly due to:
A)
diffusion of charges
done
clear
B)
depends upon the nature of material
done
clear
C)
drift of charges
done
clear
D)
both drift and diffusion of charges.
done
clear
View Answer play_arrow
question_answer 24) Two waves each of amplitude a and frequency \[f\] have a phase difference \[\frac{\pi }{2}\]The amplitude and frequency of resultant wave due to their superposition will be:
A)
\[\frac{a}{\sqrt{2}},\frac{f}{2}\]
done
clear
B)
\[\frac{a}{\sqrt{2}},f\]
done
clear
C)
\[2a,\frac{f}{2}\]
done
clear
D)
\[\sqrt{2}a,f\]
done
clear
View Answer play_arrow
question_answer 25) Two thin long parallel wires separated by a distance b are carrying a current i A each. The magnitude of the force per unit length will be:
A)
\[\frac{{{\mu }_{2}}{{i}^{2}}}{{{b}^{2}}}\]
done
clear
B)
\[\frac{{{\mu }_{0}}{{i}^{2}}}{2\pi b}\]
done
clear
C)
\[\frac{{{\mu }_{0}}i}{2\pi {{b}^{2}}}\]
done
clear
D)
\[\frac{{{\mu }_{0}}i}{2\pi b}\]
done
clear
View Answer play_arrow
question_answer 26) A particle moves in a circular orbit under the action of a central attractive force inversely proportional to distance r. The speed of the particle is:
A)
proportional to \[{{r}^{2}}\]
done
clear
B)
independent of r
done
clear
C)
proportional to r
done
clear
D)
proportional to 1/r
done
clear
View Answer play_arrow
question_answer 27) The potential energy of a particle doing S.H.M. is 2.5 J. When displacement is half of amplitude, then the total energy is:
A)
5 J
done
clear
B)
10 J
done
clear
C)
15 J
done
clear
D)
20 J
done
clear
View Answer play_arrow
question_answer 28) A diver at a depth 12m inside water (\[\mu \]= 4/3) sees the sky in a cone of semi-vertical angle:
A)
\[{{\sin }^{-1}}\left( \frac{4}{3} \right)\]
done
clear
B)
\[{{\tan }^{-1}}\left( \frac{4}{3} \right)\]
done
clear
C)
\[{{\sin }^{-1}}\left( \frac{4}{3} \right)\]
done
clear
D)
\[{{90}^{0}}\]
done
clear
View Answer play_arrow
question_answer 29) A parallel plate capacitor has capacitance C. If it is equally filled with parallel layers of materials of dielectric constants \[{{K}_{1}}\], and \[{{K}_{2}}\], its capacity becomes Q. The ratio of \[{{C}_{1}}\] to C is:
A)
\[{{k}_{1}}+{{k}_{2}}\]
done
clear
B)
\[\frac{{{k}_{1}}+{{k}_{2}}}{{{k}_{1}}+{{k}_{2}}}\]
done
clear
C)
\[\frac{{{k}_{1}}+{{k}_{2}}}{{{k}_{1}}{{k}_{2}}}\]
done
clear
D)
\[\frac{2{{k}_{1}}{{k}_{2}}}{{{k}_{1}}+{{k}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 30) An electric motor exerts a force of 40 N on a cable and pulls it by distance of 30 m in one minute. The power supplied by the motor (in watt) is:
A)
10
done
clear
B)
2
done
clear
C)
200
done
clear
D)
20
done
clear
View Answer play_arrow
question_answer 31) A stone falls reel such that the distance covered by it in the last second of its motion is equal to the distance covered by it in the first 5 seconds. It remained in air for:
A)
12 sec
done
clear
B)
13 sec
done
clear
C)
25 sec
done
clear
D)
26 sec
done
clear
View Answer play_arrow
question_answer 32) A hydrogen atom in its ground state absorbs 10.2 eV of energy. The orbital angular momentum is increased by:
A)
\[4.22\,\times {{10}^{-3}}\,J-\sec \]
done
clear
B)
\[2.11\,\times {{10}^{-34}}\,J-\sec \]
done
clear
C)
\[3.16\,\times {{10}^{-34}}\,J-\sec \]
done
clear
D)
\[1.05\,\times {{10}^{-34}}\,J-\sec \]
done
clear
View Answer play_arrow
question_answer 33) The peak value of an alternating e.m.f. E is given by \[E={{E}_{0}}\,\cos \omega t\] is 10volt and its frequency is 50 Hz. At time\[t=\frac{1}{600}\]sec, the instantaneous e.m.f. is:
A)
1 V
done
clear
B)
5 V
done
clear
C)
10 V
done
clear
D)
\[5\sqrt{3}V\]
done
clear
View Answer play_arrow
question_answer 34) Two tangent galvanometer having coils of same radius are connected in series. A current flowing in them produces deflections of \[60{}^\circ \] and \[45{}^\circ \]respectively. The ratio of number of turns in the coils is:
A)
\[\sqrt{3}/1\]
done
clear
B)
\[(\sqrt{3}+1)(\sqrt{2}-1)\]
done
clear
C)
\[(\sqrt{3}+1)/1\]
done
clear
D)
4/3
done
clear
View Answer play_arrow
question_answer 35) A proton and an \[\alpha \]-particle enter a uniform magnetic field perpendicular to y-axis with the same speed. If proton takes 25\[\mu \]sec to make 5 revolutions, then time period for the \[\alpha \]-particle would be:
A)
5\[\mu \] sec
done
clear
B)
10\[\mu \] sec
done
clear
C)
25\[\mu \] sec
done
clear
D)
50 \[\mu \] sec
done
clear
View Answer play_arrow
question_answer 36) A transmitting station transmits radio waves of wavelength 360 m. Find the inductance of the coil required with a condenser of capacity 1.20 \[\mu \]F in the resonant circuit then:
A)
\[1.25\times {{10}^{-8}}H\]
done
clear
B)
\[3.07\,\times {{10}^{-8}}\,H\]
done
clear
C)
\[2.25\,\times {{10}^{-8}}\,H\]
done
clear
D)
\[1.9\,\times {{10}^{-8}}H\]
done
clear
View Answer play_arrow
question_answer 37) Which of the following is a good nuclear fuel?
A)
Plutonium-239
done
clear
B)
Neptunium-239
done
clear
C)
Thorium-236
done
clear
D)
Uranium-236
done
clear
View Answer play_arrow
question_answer 38) Which of the following solution is the most dilute? (Volume is same in each)
A)
\[\text{0}\text{.5}\,\text{M}\,{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]
done
clear
B)
\[\text{1}\text{.2}\,\text{N}\,{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]
done
clear
C)
\[\text{48}\,\text{g/l}\text{.}\,{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]
done
clear
D)
5% \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]by weight
done
clear
View Answer play_arrow
question_answer 39) Which is the oxidant in the following reaction? \[\text{5KI}\,\text{+}\,\text{KI}{{\text{O}}_{3}}+6HCl\xrightarrow{{}}3{{I}_{2}}+6KCl+3{{H}_{2}}O\]
A)
\[{{I}_{2}}\]
done
clear
B)
\[KI{{O}_{3}}\]
done
clear
C)
\[KI\]
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 40) Which of the following is used to lower the body temperature in fever?
A)
Antimalarial
done
clear
B)
Antibiotic
done
clear
C)
Antiseptic
done
clear
D)
Antipyretic
done
clear
View Answer play_arrow
question_answer 41) \[{{\Psi }^{2}}=0\] represents the part of atom around nucleus, where probability of finding the electron is:
A)
0%
done
clear
B)
maximum possibility
done
clear
C)
95-99%
done
clear
D)
none of these.
done
clear
View Answer play_arrow
question_answer 42) An alkene, on ozonolysis gives acetaldehyde and acetone. The alkene may be:
A)
2-methyl-butene-1
done
clear
B)
2-methyl-butene-2
done
clear
C)
3-methyl-pentene-2
done
clear
D)
2-methyl-pentene-2
done
clear
View Answer play_arrow
question_answer 43) Amines cannot show:
A)
geometrical isomerism
done
clear
B)
optical isomerism
done
clear
C)
metamerism
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 44) Silicons are:
A)
halo derivatives of alkyl silanes
done
clear
B)
tetra alkyl silanes
done
clear
C)
hydrolysis product of alkyl halosilanes
done
clear
D)
polymers of halosilanes
done
clear
View Answer play_arrow
question_answer 45) The value of second ionisation energy is highest for:
A)
N
done
clear
B)
O
done
clear
C)
Be
done
clear
D)
Li
done
clear
View Answer play_arrow
question_answer 46) Acetic acid and formic acid do not differ in their action on:
A)
ammonical \[\text{AgN}{{\text{O}}_{\text{3}}}\]
done
clear
B)
\[\text{NaHC}{{\text{O}}_{\text{3}}}\]
done
clear
C)
neutral \[\text{FeC}{{\text{l}}_{3}}\]
done
clear
D)
conc. \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\]
done
clear
View Answer play_arrow
question_answer 47) Which of the following is not a colloid?
A)
Milk
done
clear
B)
Blood
done
clear
C)
Muddy water
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 48) The maximum unpaired electrons an present in:
A)
\[\text{C}{{\text{a}}^{2+}}\]
done
clear
B)
\[F{{e}^{2+}}\]
done
clear
C)
\[N{{i}^{2+}}\]
done
clear
D)
\[M{{n}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 49) Which of the following is insoluble in yellow ammonium sulphide?
A)
\[A{{s}_{2}}{{S}_{3}}\]
done
clear
B)
\[SnS\]
done
clear
C)
\[CdS\]
done
clear
D)
\[S{{b}_{2}}{{S}_{3}}\]
done
clear
View Answer play_arrow
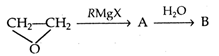
question_answer 50)
What is the product B in the reaction:
A)
\[{{1}^{o}}\] alcohol
done
clear
B)
\[{{2}^{o}}\]alcohol
done
clear
C)
\[{{3}^{o}}\]alcohol
done
clear
D)
ketone
done
clear
View Answer play_arrow
question_answer 51) What is X in the reaction \[C{{H}_{3}}CH=CH.CHO\xrightarrow{LiAl{{H}_{4}}}X\]
A)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CHO\]
done
clear
C)
\[C{{H}_{3}}CH=CHC{{H}_{2}}OH\]
done
clear
D)
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH\]
done
clear
View Answer play_arrow
question_answer 52) The diffusion rate of a gas is one fourth of that of hydrogen. The molecular weight of gas will be:
A)
16
done
clear
B)
32
done
clear
C)
64
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 53) Which of the following does not has covalent bond?
A)
\[\text{NaOH}\]
done
clear
B)
\[\text{N}{{\text{H}}_{4}}Cl\]
done
clear
C)
\[CsCl\]
done
clear
D)
\[{{H}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 54) By passage of IF of electricity, which is not obtained?
A)
1 mol of Cu
done
clear
B)
0.5 mol of Mg
done
clear
C)
9 g of Al
done
clear
D)
5.6 1 of \[{{\text{O}}_{\text{2}}}\] gas at anode
done
clear
View Answer play_arrow
question_answer 55) The conjugate acid of \[\text{HN}{{\text{O}}_{3}}\] is:
A)
\[HN{{O}_{2}}\]
done
clear
B)
\[HN{{O}_{4}}\]
done
clear
C)
\[NO_{3}^{-}\]
done
clear
D)
\[{{H}_{2}}NO_{3}^{+}\]
done
clear
View Answer play_arrow
question_answer 56) For a reaction, \[\text{A +}\,\text{B}\xrightarrow{{}}\text{P}\]the rate of reaction becomes four times, when concentration of A is increased twice and rate of reaction reduce to half when concentration of B is increased twice keeping A constant. The order of reaction is:
A)
zero
done
clear
B)
one
done
clear
C)
two
done
clear
D)
three
done
clear
View Answer play_arrow
question_answer 57) Which of the following is not a state function?
A)
Entropy
done
clear
B)
Internal energy
done
clear
C)
Heat
done
clear
D)
Free energy
done
clear
View Answer play_arrow
question_answer 58) In the case of a radio isotope, the value of \[{{\text{t}}_{1/2}}\] and \[\lambda \]are identical in magnitude. The value is:
A)
\[\frac{1}{0.693}\]
done
clear
B)
\[{{(0.693)}^{1/2}}\]
done
clear
C)
\[\frac{0.693}{2}\]
done
clear
D)
\[0.693\]
done
clear
View Answer play_arrow
question_answer 59)
The reaction,
A)
Hofmanns reaction
done
clear
B)
Schotten-Baumann reaction
done
clear
C)
Gattermanns reaction
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 60) Which metal is used in galvanization of iron?
A)
Copper
done
clear
B)
Tin
done
clear
C)
Zinc
done
clear
D)
Magnesium
done
clear
View Answer play_arrow
question_answer 61) The molecular, mass of \[\text{NaCl}\] as determined by osmotic pressure measurement is:
A)
58.5
done
clear
B)
less than 58.5
done
clear
C)
more than 58.5
done
clear
D)
unpredicitable
done
clear
View Answer play_arrow
question_answer 62) Which is not a \[\pi -\] bonded complex?
A)
Zeisses salt
done
clear
B)
Ferrocene
done
clear
C)
Dibenzene chromium
done
clear
D)
Tetraethyl lead
done
clear
View Answer play_arrow
question_answer 63) When yellow solution of \[\text{FeC}{{\text{l}}_{\text{3}}}\] is treated with \[\text{SnC}{{\text{l}}_{2}},\] the colour changes to:
A)
green
done
clear
B)
black
done
clear
C)
grey
done
clear
D)
no colour change
done
clear
View Answer play_arrow
question_answer 64) Which of the following, follows the octet rule?
A)
\[B{{F}_{3}}\]
done
clear
B)
\[{{N}_{2}}\]
done
clear
C)
\[PC{{l}_{5}}\]
done
clear
D)
\[S{{F}_{6}}\]
done
clear
View Answer play_arrow
question_answer 65) Ethanamide on treatment with \[\text{B}{{\text{r}}_{\text{2}}}\text{/NaOH,}\] gives:
A)
ethanamine
done
clear
B)
ethyl alcohol
done
clear
C)
methanamine
done
clear
D)
bromoform
done
clear
View Answer play_arrow
question_answer 66) What is the product \[\Upsilon \] in the reaction, \[C{{H}_{2}}=CH.CHO\xrightarrow{LiAl{{H}_{4}}}X\xrightarrow{alk.KMn{{O}_{4}}}\Upsilon \]
A)
\[C{{H}_{3}}C{{H}_{2}}.COOH\]
done
clear
B)
\[C{{H}_{2}}OH.C{{H}_{2}}OH\]
done
clear
C)
\[C{{H}_{2}}OH-CHOH-C{{H}_{2}}OH\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 67) The product \[\Upsilon \] in the reaction, is: \[C{{H}_{3}}-C\equiv CH\xrightarrow{2HBr}X\xrightarrow{aq.NaOH}\Upsilon \]
A)
\[C{{H}_{3}}CHO\]
done
clear
B)
\[~C{{H}_{2}}C{{H}_{2}}COOH\]
done
clear
C)
\[C{{H}_{3}}C{{H}_{2}}CHO\]
done
clear
D)
\[C{{H}_{3}}COC{{H}_{3}}\]
done
clear
View Answer play_arrow
question_answer 68) The correct order of increasing electron affinity, is:
A)
\[Be<B<N<C\]
done
clear
B)
\[Be<B<C<N\]
done
clear
C)
\[N<C<B<Be\]
done
clear
D)
\[N<C<Be<B\]
done
clear
View Answer play_arrow
question_answer 69) pH of \[{{10}^{-7}}\text{M NaOH}\] is:
A)
7
done
clear
B)
7.3010
done
clear
C)
6.6990
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 70) For the reaction, \[CO(g)+{{H}_{2}}O(g)\rightleftharpoons C{{O}_{2}}(g)+{{H}_{2}}(g)\] at \[\text{200}{{\,}^{\text{o}}}\text{C,}\,\,{{P}_{C{{O}_{2}}}}=0.2\,\text{atm}\text{.,}\,\,{{\text{P}}_{{{\text{H}}_{2}}}}=0.1\,\text{atm}\text{.,}\] \[{{\text{P}}_{\text{CO}}}\text{=}\,\text{2}\,\text{atm}\text{.,}\]and \[{{\text{P}}_{{{\text{H}}_{2}}O}}=0.01\,\text{atm}\text{.}\]The value of \[{{K}_{c}}\] will be:
A)
1.2
done
clear
B)
1.0
done
clear
C)
0.028
done
clear
D)
2.42
done
clear
View Answer play_arrow
question_answer 71) The weight of 1 curie \[{{\,}_{82}}P{{b}^{214}}({{t}_{1/2}}=26.8\,\min .)\] in grams is:
A)
\[3.1\times {{10}^{-8}}g\]
done
clear
B)
\[1.55\times {{10}^{-8}}\,g\]
done
clear
C)
\[6.2\times {{10}^{-8}}g\]
done
clear
D)
\[3.1\times {{10}^{-10}}g\]
done
clear
View Answer play_arrow
question_answer 72) The entropy change of water, when 18 g water is converted at constant temperature into steam, is: (Given, \[\Delta {{H }_{\upsilon }}=40.7\text{kJ}\text{mo}{{\text{l}}^{-1}}\]):
A)
\[109.11\,\text{J}{{\text{K}}^{-1}}\text{mo}{{\text{l}}^{-1}}\]
done
clear
B)
\[218.22\,\text{J}{{\text{K}}^{-1}}\text{mo}{{\text{l}}^{-1}}\]
done
clear
C)
\[54.56\,\text{J}{{\text{K}}^{-1}}\,\text{mo}{{\text{l}}^{-1}}\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 73) Which is not an ore of magnesium?
A)
Dolamite
done
clear
B)
Carnallite
done
clear
C)
Felspar
done
clear
D)
Sorels cement
done
clear
View Answer play_arrow
question_answer 74) The correct de-Broglie equation is:
A)
\[\lambda =\frac{h}{m\upsilon }\]
done
clear
B)
\[\lambda =\frac{m\upsilon }{h}\]
done
clear
C)
\[\lambda =\frac{mh}{\upsilon }\]
done
clear
D)
\[\lambda =\frac{h\upsilon }{m}\]
done
clear
View Answer play_arrow
question_answer 75) Which of the following is not a polymer?
A)
DNA
done
clear
B)
Teflon
done
clear
C)
Freon
done
clear
D)
Gutta purcha
done
clear
View Answer play_arrow
question_answer 76) Which of the following plant is generally described as a living fossil?
A)
Cycas
done
clear
B)
Cupressus
done
clear
C)
Taxus
done
clear
D)
Ephedra
done
clear
View Answer play_arrow
question_answer 77) Entamoeba histolytica is:
A)
found in intestine
done
clear
B)
found in liver
done
clear
C)
found in. coelom
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 78) The base of oxysome is also called as:
A)
\[{{F}_{5}}\] particle
done
clear
B)
\[{{F}_{6}}\] particle
done
clear
C)
\[{{F}_{1}}\] particle
done
clear
D)
\[{{F}_{0}}\] particle
done
clear
View Answer play_arrow
question_answer 79) Phosphate pollution is caused by:
A)
phosphate rock only
done
clear
B)
agriculture fertilizers only
done
clear
C)
sewage and phosphate rock
done
clear
D)
sewage and agriculture fertilizers
done
clear
View Answer play_arrow
question_answer 80) Angiosperms, to which the largest flower belongs, is:
A)
total stem parasite
done
clear
B)
partial stem parasite
done
clear
C)
total root parasite
done
clear
D)
partial root parasite
done
clear
View Answer play_arrow
question_answer 81) Heterospory, seed habit is often exhibited by a plant possessing:
A)
bract
done
clear
B)
spathe
done
clear
C)
petiole
done
clear
D)
ligule
done
clear
View Answer play_arrow
question_answer 82) The codons causing chain termination are:
A)
TAG, TAA, TGA
done
clear
B)
GAT, AAT, AGT
done
clear
C)
AGT, TAG, UGA
done
clear
D)
UAG, UGA, UAA
done
clear
View Answer play_arrow
question_answer 83) Edible part in litchi is:
A)
mesocarp
done
clear
B)
fleshy aril
done
clear
C)
endosperm
done
clear
D)
pericarp
done
clear
View Answer play_arrow
question_answer 84) Which of the following are homologous organs?
A)
Wings of bird and wings of insect
done
clear
B)
Wings of bat and wings of cockroach
done
clear
C)
Wings of bird and hand of human
done
clear
D)
Nails of human being and claws in animals
done
clear
View Answer play_arrow
question_answer 85) In human beings, multiple genes are involved in the inheritance of:
A)
colour blindness
done
clear
B)
phenylketonuria
done
clear
C)
sickle cell anaemia
done
clear
D)
skin colour
done
clear
View Answer play_arrow
question_answer 86) Which of the following plant kingdom is called amphibians?
A)
Tracheophyta
done
clear
B)
Bryophyta
done
clear
C)
Pteridophyta
done
clear
D)
Thallophyta
done
clear
View Answer play_arrow
question_answer 87) Model for DNA structure was proposed by:
A)
Beadle and Tatum
done
clear
B)
M. C. Chung
done
clear
C)
Purkinje
done
clear
D)
Watson and Crick
done
clear
View Answer play_arrow
question_answer 88) Cell wall is outer covering of plant cell made up of cellulose it is:
A)
semipermeable
done
clear
B)
permeable
done
clear
C)
non permeable
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 89) Protein is the body building material of animals. It is the polymer of:
A)
glucose
done
clear
B)
nucleotides
done
clear
C)
amino acids
done
clear
D)
fatty acids
done
clear
View Answer play_arrow
question_answer 90) A molecule of sedoheptulose has carbon atoms numbering:
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
7
done
clear
View Answer play_arrow
question_answer 91) Which of the following disease is the result of thiamine deficiency?
A)
Marasmus
done
clear
B)
Beri-beri
done
clear
C)
Rickets
done
clear
D)
Kwashiorkor
done
clear
View Answer play_arrow
question_answer 92) Chemically wax is:
A)
A protein
done
clear
B)
A lipid
done
clear
C)
A carbohydrate
done
clear
D)
An amino acid
done
clear
View Answer play_arrow
question_answer 93) The chemical formula of chlorophyll-b is:
A)
\[{{C}_{55}}{{H}_{72}}\,{{O}_{5}}{{N}_{5}}Mg\]
done
clear
B)
\[{{C}_{55}}{{H}_{72}}\,{{O}_{5}}{{N}_{4}}Mg\]
done
clear
C)
\[{{C}_{55}}{{H}_{70}}\,{{O}_{6}}{{N}_{4}}Mg\]
done
clear
D)
\[{{C}_{55}}{{H}_{70}}\,{{O}_{5}}{{N}_{5}}Mg\]
done
clear
View Answer play_arrow
question_answer 94) The reaction of glycolysis occurs in:
A)
mitochondria
done
clear
B)
ribosome
done
clear
C)
cytoplasm
done
clear
D)
golgi complex
done
clear
View Answer play_arrow
question_answer 95) In a human being the number of cranial nerves are:
A)
10 pairs
done
clear
B)
12 pairs
done
clear
C)
6 pairs
done
clear
D)
20 pairs
done
clear
View Answer play_arrow
question_answer 96) The plants respond to photo-periods due to the presence of:
A)
phytochromes
done
clear
B)
stomata
done
clear
C)
enzymes
done
clear
D)
phytohormones
done
clear
View Answer play_arrow
question_answer 97) The pioneers in the field of organic evolution are:
A)
Darwin, Lamarck, Hugo de Vries, Huxley
done
clear
B)
Karl Landsteiner, Hugo de Vries, Malthus, Darwin
done
clear
C)
Lamarck, Karl Landsteiner, Malthus, Hungo de Vries
done
clear
D)
Darwin, Lamarck, Karl Landsteiner, Hugo de Vries
done
clear
View Answer play_arrow
question_answer 98) The cell wall of bacteria is composed of:
A)
murein
done
clear
B)
chitin
done
clear
C)
cellulose
done
clear
D)
suberin
done
clear
View Answer play_arrow
question_answer 99) The phenomenon, in which an allele of one gene suppresses the activity of an allele of another gene, is known as:
A)
suppression
done
clear
B)
inactivation
done
clear
C)
epistasis
done
clear
D)
dominance
done
clear
View Answer play_arrow
question_answer 100) What is common among amylase, rennin and trypsin?
A)
These all are proteins
done
clear
B)
These all are proteolytic enzymes
done
clear
C)
These are produced in stomach
done
clear
D)
These all are hormones
done
clear
View Answer play_arrow
question_answer 101) The vagus nerve is the cranial nerve numbering:
A)
10th
done
clear
B)
9th
done
clear
C)
7th
done
clear
D)
5th
done
clear
View Answer play_arrow
question_answer 102) The kidney of an adult frog is:
A)
pronephros
done
clear
B)
mesonephros
done
clear
C)
metanephros
done
clear
D)
opisthonephros
done
clear
View Answer play_arrow
question_answer 103) Which of the following is free living aerobic, non-photosynthetic nitrogen fixing bacterium?
A)
Rhizobium
done
clear
B)
Azotobacter
done
clear
C)
Nostoc
done
clear
D)
Azospirillium
done
clear
View Answer play_arrow
question_answer 104) Is a person shows production of interferons in his body the chances are that he has got an infection of:
A)
typhoid
done
clear
B)
measles
done
clear
C)
tetanus
done
clear
D)
malaria
done
clear
View Answer play_arrow
question_answer 105) Centromere is a part of:
A)
ribosomes
done
clear
B)
mitochondria
done
clear
C)
chromosome
done
clear
D)
endoplasmic reticulum
done
clear
View Answer play_arrow
question_answer 106) \[NADP{{H}_{2}}\] is generated through:
A)
photosystem I
done
clear
B)
photosystem II
done
clear
C)
anaerobic respiration
done
clear
D)
glycolysis
done
clear
View Answer play_arrow
question_answer 107) If an angiospermic male plant is diploid and female plant tetraploid, the ploidy level of endosperm will be:
A)
haploid
done
clear
B)
triploid
done
clear
C)
tetraploid
done
clear
D)
pentaploid
done
clear
View Answer play_arrow
question_answer 108) The cheapest source of high energy among fruit crop of India is:
A)
guava
done
clear
B)
apple
done
clear
C)
banana
done
clear
D)
mango
done
clear
View Answer play_arrow
question_answer 109) In soil, water available for plants is:
A)
capillary water
done
clear
B)
hygroscopic water
done
clear
C)
gravitational water
done
clear
D)
chemically bound water
done
clear
View Answer play_arrow
question_answer 110) Brown algae is characterized by the presence of:
A)
phycocyanin
done
clear
B)
phycoerythrin
done
clear
C)
fucoxanthin
done
clear
D)
haematochrome
done
clear
View Answer play_arrow
question_answer 111) Biofertilizers are:
A)
cow dung manure and farmyard waste
done
clear
B)
quick growing crop ploughed under soil
done
clear
C)
Anabaena and Azolla
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 112) Net gain of ATP molecules, during aerobic respiration, is:
A)
30 molecules
done
clear
B)
38 molecules
done
clear
C)
40 molecules
done
clear
D)
48 molecules
done
clear
View Answer play_arrow
question_answer 113) The Nissls granules of nerves cell are made up of:
A)
ribosome
done
clear
B)
protein
done
clear
C)
DNA
done
clear
D)
RNA
done
clear
View Answer play_arrow
question_answer 114) In ureotelic animals urea is formed by:
A)
Ornithine cycle
done
clear
B)
Coris cycle
done
clear
C)
Krebs cycle
done
clear
D)
EMP pathway
done
clear
View Answer play_arrow
question_answer 115) The water vascular system performs all but not one function which is that:
A)
excretion
done
clear
B)
respiration
done
clear
C)
food capturing
done
clear
D)
hormone secretion
done
clear
View Answer play_arrow
question_answer 116) Which is not a fish?
A)
Scoliodon
done
clear
B)
Electric ray
done
clear
C)
Whale
done
clear
D)
Sea horse
done
clear
View Answer play_arrow
question_answer 117) Hydra does not perform locomotion by:
A)
looping
done
clear
B)
somersaulting
done
clear
C)
jet propulsion
done
clear
D)
walking
done
clear
View Answer play_arrow
question_answer 118) Which one is not the larval stage of Fascicla?
A)
Cercaria
done
clear
B)
Miracidium
done
clear
C)
Crysticercus
done
clear
D)
Redia
done
clear
View Answer play_arrow
question_answer 119) The main function of flame cells is:
A)
excretion
done
clear
B)
osmoregulation
done
clear
C)
secretion of toxins
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 120) Which part of an active enzyme is denatured by heat:
A)
apoenzyme
done
clear
B)
coenzyme
done
clear
C)
activator
done
clear
D)
holoenzyme
done
clear
View Answer play_arrow
question_answer 121) Secretion of digestive juice in stomach is from:
A)
Brunners gland
done
clear
B)
gastric gland
done
clear
C)
foveola
done
clear
D)
sub mucosal gland
done
clear
View Answer play_arrow
question_answer 122) Which one is not involved in the immune system?
A)
B-cells
done
clear
B)
T-cells
done
clear
C)
Macrophage
done
clear
D)
Erythrocytes
done
clear
View Answer play_arrow
question_answer 123) In man yellow colour of faeces is due to:
A)
pigments produced by the breakdown of haemoglobin
done
clear
B)
undigested fat which is yellow coloured
done
clear
C)
bile juice
done
clear
D)
pancreatic juice
done
clear
View Answer play_arrow
question_answer 124) Which of the following is not a function of erythrocyte?
A)
Oxygen transport
done
clear
B)
\[C{{O}_{2}}\] transport
done
clear
C)
Carriers of antigen
done
clear
D)
Phagocytosis
done
clear
View Answer play_arrow
question_answer 125) Relaxin hormone in mammals is secreted by:
A)
placenta
done
clear
B)
follicle
done
clear
C)
ovary
done
clear
D)
testis
done
clear
View Answer play_arrow
question_answer 126) Motile sperms are absent in:
A)
Mitcor
done
clear
B)
Cycas
done
clear
C)
Chlorella
done
clear
D)
Fitnaria
done
clear
View Answer play_arrow
question_answer 127) Lomasomes are present in:
A)
protozoan
done
clear
B)
fungi
done
clear
C)
bacteria
done
clear
D)
virus
done
clear
View Answer play_arrow
question_answer 128) Tungro disease affects:
A)
wheat
done
clear
B)
barley
done
clear
C)
rice
done
clear
D)
sorghum
done
clear
View Answer play_arrow
question_answer 129) Mycorrhizae is a good example of:
A)
symbiosis
done
clear
B)
commensalism
done
clear
C)
protocooperation
done
clear
D)
competition
done
clear
View Answer play_arrow
question_answer 130) Imperfect fungi are known as:
A)
Ascomycetes
done
clear
B)
Deuteromycetes
done
clear
C)
Basidiomycetes
done
clear
D)
Phycomycetes
done
clear
View Answer play_arrow
question_answer 131) Which acid is not produced by various species of fungi?
A)
Gluconic acid
done
clear
B)
Acetic acid
done
clear
C)
Sulphuric acid
done
clear
D)
Succinic acid
done
clear
View Answer play_arrow
question_answer 132) FAD coenzyme is made from:
A)
pantothenic acid
done
clear
B)
thymine
done
clear
C)
riboflavin
done
clear
D)
folic acid
done
clear
View Answer play_arrow
question_answer 133) Which of the following is not photoautotroph?
A)
Purple sulphur bacteria
done
clear
B)
Purple non-sulphur bacteria
done
clear
C)
Green sulphur bacteria
done
clear
D)
Cyanobacteria
done
clear
View Answer play_arrow
question_answer 134) Bacterial chlorophyll absorbs mainly:
A)
infra red light
done
clear
B)
visible light
done
clear
C)
UV rays
done
clear
D)
X-rays
done
clear
View Answer play_arrow
question_answer 135) Cell wall of red algae contains:
A)
cellulose
done
clear
B)
cellulose + alginic acid
done
clear
C)
cellulose + pectin
done
clear
D)
no cell wall
done
clear
View Answer play_arrow
question_answer 136) Process in which the phosphate group of a compound is removed and directly added to ADP is:
A)
substrate level phosphorylation
done
clear
B)
photophosphorylation
done
clear
C)
oxidative phosphorylation
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 137) AIDS is confirmed by:
A)
ELISA
done
clear
B)
Western blot
done
clear
C)
Northern blot
done
clear
D)
Southern blot
done
clear
View Answer play_arrow
question_answer 138) The vitamin responsible for antisterile activity is:
A)
vitamin B
done
clear
B)
vitamin E
done
clear
C)
vitamin K
done
clear
D)
vitamin H
done
clear
View Answer play_arrow
question_answer 139) EEG is done to detect:
A)
heart disorders
done
clear
B)
brain disorders
done
clear
C)
liver disorders
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 140) Which of the following is a banned pesticide?
A)
DDT
done
clear
B)
CAN
done
clear
C)
EDB
done
clear
D)
DNB
done
clear
View Answer play_arrow
question_answer 141) Leaf lamina is modified into two toothed jaws in:
A)
Nepenthes
done
clear
B)
Dionaca
done
clear
C)
Drosera
done
clear
D)
Utricularia
done
clear
View Answer play_arrow
question_answer 142) Name the hydrophyte which is not free floating?
A)
Wolffia
done
clear
B)
Lenina
done
clear
C)
Utricularia
done
clear
D)
Eichhornia
done
clear
View Answer play_arrow
question_answer 143) Root cap is not generally present in:
A)
mesophytes
done
clear
B)
halophytes
done
clear
C)
hydrophytes
done
clear
D)
xerophytes
done
clear
View Answer play_arrow
question_answer 144) Which is not a flightless bird?
A)
Rhea
done
clear
B)
Dromeaus
done
clear
C)
Apteryx
done
clear
D)
Columba
done
clear
View Answer play_arrow
question_answer 145) When the sepals or petals meet by their edges without overlapping the aestivation is:
A)
imbricate
done
clear
B)
valvate
done
clear
C)
twisted
done
clear
D)
axillary
done
clear
View Answer play_arrow
question_answer 146) Boat shaped anterior petal in garden pea is called:
A)
wing
done
clear
B)
alae
done
clear
C)
keel
done
clear
D)
ship
done
clear
View Answer play_arrow
question_answer 147) The process of formation of glucose from non carbohydrate sources is called:
A)
glycogenesis
done
clear
B)
glycogenolysis
done
clear
C)
glyconeogenesis
done
clear
D)
glycolysis
done
clear
View Answer play_arrow
question_answer 148) Northern blotting is used for:
A)
DNA transfer
done
clear
B)
RNA transfer
done
clear
C)
protein transfer
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 149) Heterocysts are found in:
A)
Aspergillus
done
clear
B)
Nostoc
done
clear
C)
Cystopus
done
clear
D)
Ulothrix
done
clear
View Answer play_arrow
question_answer 150) Synthetic vaccines are called:
A)
first generation
done
clear
B)
second generation
done
clear
C)
third generation
done
clear
D)
none of these
done
clear
View Answer play_arrow
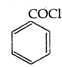 \[+\,{{C}_{2}}{{H}_{2}}OH+NaOH\xrightarrow{{}}\]
\[+\,{{C}_{2}}{{H}_{2}}OH+NaOH\xrightarrow{{}}\] 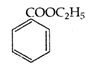 \[+NaCl+{{H}_{2}}\]is called
\[+NaCl+{{H}_{2}}\]is called
