question_answer 1) A weight w is suspended from the midpoint of a rope, whose ends are at the same level. In order to make the rope perfectly horizontal, the force applied to each of its ends must be:
A)
less than w
done
clear
B)
equal to w
done
clear
C)
equal to 2w
done
clear
D)
infinitely large
done
clear
View Answer play_arrow
question_answer 2) The velocity of a particle at an instant is 10 m/s. After 3s its velocity will become 16 m/s. The velocity at 2 s, before the given instant will be:
A)
6 m/s
done
clear
B)
4 m/s
done
clear
C)
2 m/s
done
clear
D)
1 m/s
done
clear
View Answer play_arrow
question_answer 3) A heavy stone hanging from a massless string of length 15 m is projected horizontally with speed 147 m/s. The speed of the particle at the point where the tension in the string equals the weight of the particle is?
A)
10 m/s
done
clear
B)
7 m/s
done
clear
C)
12 m/s
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 4) When a body moves with constant speed in a circular path, then:
A)
work done will be zero
done
clear
B)
acceleration will be zero
done
clear
C)
no force acts on the body
done
clear
D)
its velocity remains constant
done
clear
View Answer play_arrow
question_answer 5) A body initially at rest is moving with uniform acceleration a Its velocity after n second is v. The displacement of the body in 2 s is
A)
\[\frac{2v(n-1)}{n}\]
done
clear
B)
\[\frac{v(n-1)}{n}\]
done
clear
C)
\[\frac{v(n+1)}{n}\]
done
clear
D)
\[\frac{2v(n+1)}{n}\]
done
clear
View Answer play_arrow
question_answer 6) The magnetic force on a point charge is \[\vec{F}=q\,(\vec{v}\times \vec{B})\]Here, q = electric charge \[\vec{V}\] = velocity of point charge \[\vec{B}\] = magnetic field The dimensions of \[\vec{B}\] is
A)
\[\left[ ML{{T}^{-1}}A \right]\]
done
clear
B)
\[[{{M}^{2}}L{{T}^{-2}}\,{{A}^{-1}}]\]
done
clear
C)
\[\left[ M{{L}^{-2}}{{A}^{-1}} \right]\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 7) The first diffraction minimum due to single slit diffraction is \[\theta \], for a light of wave-length 5000\[\overset{0}{\mathop{A}}\,\]. If the width of the slit is \[1\times {{10}^{-4}}\,cm,\] then the value of \[\theta \]is:
A)
\[30{}^\circ \]
done
clear
B)
\[45{}^\circ \]
done
clear
C)
\[60{}^\circ \]
done
clear
D)
\[15{}^\circ \]
done
clear
View Answer play_arrow
question_answer 8) A stone of mass m tied to a string of length I is rotated in a circle with the other end of the string as the centre. The speed of the stone is v. If the string breaks, the stone will:
A)
move towards the centre
done
clear
B)
move away from the centre
done
clear
C)
move along tangent
done
clear
D)
stop
done
clear
View Answer play_arrow
question_answer 9) A body of mass 2 kg is placed on rough horizontal plane. The coefficient of friction between body and plane is 0.2. Then:
A)
body will move in forward direction if F = 5N
done
clear
B)
body will move in backward direction with acceleration \[0.5\,\,m/{{s}^{2}},\] if force F = 3 N
done
clear
C)
If F = 3 N, then body will be in rest condition
done
clear
D)
both (a) and (c) are correct
done
clear
View Answer play_arrow
question_answer 10) A particle moves with a velocity \[(5\mathbf{\hat{i}}-3\mathbf{\hat{j}}+6\mathbf{\hat{k}})\]m/s under the influence of a constant force \[\vec{F}=(10\mathbf{\hat{i}}+10\mathbf{\hat{j}}+12\mathbf{\hat{k}})\]N. The instantaneous power applied to the particle is:
A)
200 J/s
done
clear
B)
40 J/s
done
clear
C)
140 J/s
done
clear
D)
170 J/s
done
clear
View Answer play_arrow
question_answer 11) The potential energy of a particle of mass 5 kg moving in the x - y plane is given by \[U=(-7x+24y)\,J,\,x\] and y being in metre. If the particle starts from rest from origin, then speed of particle at t = 2 s is:
A)
5 m/s
done
clear
B)
14 m/s
done
clear
C)
17.5 m/s
done
clear
D)
10 m/s
done
clear
View Answer play_arrow
question_answer 12) The ratio of radii of gyration of a circular disc and a circular ring of the same radii and same mass about a tangential axis in the plane is:
A)
1 : 2
done
clear
B)
\[\sqrt{5}\,:\sqrt{6}\]
done
clear
C)
2 : 3
done
clear
D)
2 : 1
done
clear
View Answer play_arrow
question_answer 13) A particle performs uniform circular motion with an angular momentum L. If the frequency of particle motion is doubled and its KE is halved, the angular momentum becomes:
A)
2 L
done
clear
B)
4 L
done
clear
C)
\[\frac{L}{2}\]
done
clear
D)
\[\frac{L}{4}\]
done
clear
View Answer play_arrow
question_answer 14) The minimum energy required to launch a m kg satellite from earths surface in a circular orbit at an altitude of 2R, R is the radius of earth, will be:
A)
3 mgR
done
clear
B)
\[\frac{5}{6}mgR\]
done
clear
C)
\[2mgR\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 15) A satellite is moving on a circular path of radius r around the earth has a time period T. If its radius slightly increases by\[\Delta r\] the change in its time period is
A)
\[\frac{3}{2}\left( \frac{T}{r} \right)\Delta r\]
done
clear
B)
\[\left( \frac{T}{r} \right)\Delta r\]
done
clear
C)
\[\frac{3}{2}\left( \frac{{{T}^{2}}}{{{r}^{2}}} \right)\Delta r\]
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 16) A particle executes SHM, its time period is 16 s. If it passes through the centre of oscillation then its velocity is 2 m/s at time 2 s. The amplitude will be:
A)
7.2 m
done
clear
B)
4 cm
done
clear
C)
6 cm
done
clear
D)
0.72 m
done
clear
View Answer play_arrow
question_answer 17) A simple harmonic oscillator has amplitude A, angular velocity\[\omega \], and mass m. Then average energy in one time period will be:
A)
\[\frac{1}{4}m{{\omega }^{2}}{{A}^{2}}\]
done
clear
B)
\[\frac{1}{2}m{{\omega }^{2}}{{A}^{2}}\]
done
clear
C)
\[m{{\omega }^{2}}{{A}^{2}}\]
done
clear
D)
0
done
clear
View Answer play_arrow
question_answer 18) The wave front due to infinity is:
A)
spherical
done
clear
B)
cylindrical
done
clear
C)
planar
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 19) An astronaut is approaching the moon. He sends out a radio signal of frequency 5000 MHz and the frequency of echo is different from that of the original frequency by 100 kHz. Hi velocity of approach with respect to the moon is:
A)
2 km/s
done
clear
B)
3 km/s
done
clear
C)
4 km/s
done
clear
D)
5 km/s
done
clear
View Answer play_arrow
question_answer 20) When a sphere is taken to bottom of sea 1 km deep, it contracts by 0.01%. The bulk modulus of elasticity of the material of sphere is :(Given : Density of water \[=1\,g/\,c{{m}^{3}}\])
A)
\[9.8\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
B)
\[10.2\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
C)
\[0.98\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
D)
\[8.4\times {{10}^{10}}N/{{m}^{2}}\]
done
clear
View Answer play_arrow
question_answer 21) The temperature of \[{{H}_{2}}\] at which the rms velocity of its molecules is seven times the rms velocity of the molecules of nitrogen at 300 K is:
A)
2100 K
done
clear
B)
1700 K
done
clear
C)
1350 K
done
clear
D)
1050 K
done
clear
View Answer play_arrow
question_answer 22) Gas exerts pressure on the walls of the container because:
A)
gas has weight
done
clear
B)
gas molecules have momentum
done
clear
C)
gas molecules collide with each other
done
clear
D)
gas molecules collide with the walls of the container
done
clear
View Answer play_arrow
question_answer 23) The inside and outside temperatures of a refrigerator are 273 K and 303 K respectively. Assuming that refrigerator cycle is reversible, for every joule of work done, the heat delivered to the surrounding will be :
A)
10 J
done
clear
B)
20 J
done
clear
C)
30 J
done
clear
D)
50 J
done
clear
View Answer play_arrow
question_answer 24) In an energy recycling process, X g of steam at \[100{}^\circ C\] becomes water at \[100{}^\circ C\] which converts Y g of ice at \[0{}^\circ C\] into water at \[100{}^\circ C\]. The ratio of X and Y will be:
A)
\[\frac{1}{3}\]
done
clear
B)
\[\frac{2}{3}\]
done
clear
C)
3
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 25) The surface temperature of the sun is T K and the solar constant for a plate is S. The sun subtends an angle \[\theta \] at the planet. Then:
A)
\[S\propto {{T}^{4}}\]
done
clear
B)
\[S\propto {{T}^{2}}\]
done
clear
C)
\[S\propto {{\theta }^{2}}\]
done
clear
D)
\[S\propto \theta \]
done
clear
View Answer play_arrow
question_answer 26) A body at a temperature of \[728{}^\circ C\] and has surface area \[5\,\,c{{m}^{2}},\] radiates 300 J of energy each minute. The emissivity is : (Given : Boltzmann constant \[=5.67\,\times {{10}^{-8}}\,W\,{{m}^{2}}{{K}^{4}}\])
A)
e = 0.18
done
clear
B)
e = 0.02
done
clear
C)
e = 0.2
done
clear
D)
e = 0.15
done
clear
View Answer play_arrow
question_answer 27) If at NTP velocity of sound in a gas is 1150 m/s, then the rms velocity of gas molecules at NTP is : (Given : R = 8.3 J/mol/K, Cp = 4.8 cal/mol/K)
A)
1600 m/s
done
clear
B)
1532.19 m/s
done
clear
C)
160 m/s
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 28) If \[\sigma =\]surface charge density, \[\varepsilon \]= electric permittivity, the dimensions of \[\frac{\sigma }{e}\] are same as:
A)
electric force
done
clear
B)
electric field intensity
done
clear
C)
pressure
done
clear
D)
electric charge
done
clear
View Answer play_arrow
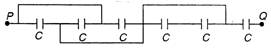
question_answer 29)
For circuit the equivalent capacitance between P and Q is:
A)
6 C
done
clear
B)
4 C
done
clear
C)
\[\frac{3C}{2}\]
done
clear
D)
\[\frac{6C}{11}\]
done
clear
View Answer play_arrow
question_answer 30) A wire has resistance \[12\Omega \]. It is bent in the form of a circle. The effective resistance between the two points on any diameter of the circle is:
A)
12\[\Omega \]
done
clear
B)
24\[\Omega \]
done
clear
C)
60\[\Omega \]
done
clear
D)
3\[\Omega \]
done
clear
View Answer play_arrow
question_answer 31) If two identical heaters each rated as (1000 W.220 V) are connected in parallel to 220 V, then the total power consumed is:
A)
200 W
done
clear
B)
2500 W
done
clear
C)
250 W
done
clear
D)
2000 W
done
clear
View Answer play_arrow
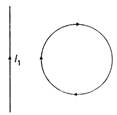
question_answer 32)
In the given figure, the loop is fixed but straight wire can move. The straight wire will:
A)
remain stationary
done
clear
B)
move towards the loop
done
clear
C)
move away from the loop
done
clear
D)
rotates about the axis
done
clear
View Answer play_arrow
question_answer 33) At a point on the right bisector of a magnetic dipole, the magnetic :
A)
potential varies as \[\frac{1}{{{r}^{2}}}\]
done
clear
B)
potential is zero at all points on the right bisector
done
clear
C)
field varies as \[{{r}^{2}}\]
done
clear
D)
field is perpendicular to the axis of dipole
done
clear
View Answer play_arrow
question_answer 34) The couple acting on a magnet of length 10 cm and pole strength 15 Am, kept in a field of \[B=2\times {{10}^{-5}}\,T,\] at an angle of 30°, is:
A)
\[1.5\times {{10}^{-5}}Nm\]
done
clear
B)
\[1.5\times {{10}^{-3}}Nm\]
done
clear
C)
\[1.5\times {{10}^{-2}}Nm\]
done
clear
D)
\[1.5\times {{10}^{-6}}Nm\]
done
clear
View Answer play_arrow
question_answer 35) An AC is represented by e = 220 sin (100\[\pi \])t V and is applied over a resistance of 110\[\Omega \]. The heat produced in 7 min is:
A)
\[11\times {{10}^{3}}\,cal\]
done
clear
B)
\[22\times {{10}^{3}}cal\]
done
clear
C)
\[33\times {{10}^{3}}cal\]
done
clear
D)
\[25\times {{10}^{3}}cal\]
done
clear
View Answer play_arrow
question_answer 36) The wave length of a radio wave of frequency of 1 MHz is:
A)
400 m
done
clear
B)
300 m
done
clear
C)
350 m
done
clear
D)
200 m
done
clear
View Answer play_arrow
question_answer 37) The correct option, if speed of gamma rays, x-rays and micro waves are \[{{v}_{g}},\,{{v}_{x}}\] and \[{{v}_{m}}\] respectively will be:
A)
\[{{v}_{g}}>{{v}_{x}}>{{v}_{m}}\]
done
clear
B)
\[{{v}_{g}}<{{v}_{x}}<{{v}_{m}}\]
done
clear
C)
\[{{v}_{g}}>{{v}_{x}}>{{v}_{m}}\]
done
clear
D)
\[{{v}_{g}}={{v}_{x}}={{v}_{m}}\]
done
clear
View Answer play_arrow
question_answer 38) The electric field E and magnetic field B in electromagnetic waves are:
A)
parallel to each other
done
clear
B)
inclined at an angle of 45°
done
clear
C)
perpendicular to each other
done
clear
D)
opposite to each other
done
clear
View Answer play_arrow
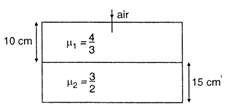
question_answer 39)
Considering normal incidence of ray, the equivalent refractive index of combination of two slabs shown in figure is:
A)
1.8
done
clear
B)
1.43
done
clear
C)
2
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 40) In Youngs double slit experiment, the spacing between the slits is d and wavelength of light used is 6000\[\overset{0}{\mathop{A}}\,\]. If the angular width of a fringe formed on a distant screen is \[1{}^\circ \], then value of d is:
A)
1 mm
done
clear
B)
0.05 mm
done
clear
C)
0.03 mm
done
clear
D)
0.01 mm
done
clear
View Answer play_arrow
question_answer 41) Polarization of light proves:
A)
corpuscular nature of light
done
clear
B)
quantum nature of light
done
clear
C)
transverse wave nature of light
done
clear
D)
longitudinal wave nature of light
done
clear
View Answer play_arrow
question_answer 42) Three particles having charges in the ratio of 2:3:5, produce the same point on the photographic film in Thomson experiment. Their masses are in the ratio of:
A)
2 : 3 : 5
done
clear
B)
5 : 3 : 2
done
clear
C)
15 : 10 : 6
done
clear
D)
3 : 5 : 2
done
clear
View Answer play_arrow
question_answer 43) In terms of Rydberg constant R, the wavenumber of the first Balmer line is:
A)
R
done
clear
B)
3R
done
clear
C)
\[\frac{5R}{36}\]
done
clear
D)
\[\frac{8R}{9}\]
done
clear
View Answer play_arrow
question_answer 44) In nuclear reaction \[_{2}H{{e}^{4}}{{+}_{z}}{{X}^{A}}{{\to }_{Z+2}}{{Y}^{A+3}}{{+}_{z}}{{M}^{A}}\] where, M denotes
A)
electron
done
clear
B)
positron
done
clear
C)
proton
done
clear
D)
neutron
done
clear
View Answer play_arrow
question_answer 45) n-alpha particles per second are emitted from N atoms of a radioactive element. The half-life of radioactive element is:
A)
\[\frac{n}{N}s\]
done
clear
B)
\[\frac{N}{n}s\]
done
clear
C)
\[\frac{0.693N}{n}s\]
done
clear
D)
\[\frac{0.693n}{N}s\]
done
clear
View Answer play_arrow
question_answer 46) Assertion: If the ice on the polar caps of the earth melts, then length of day will increase. Reason: Moment of inertia of earth increases, as ice on polar caps melts.
A)
If both assertion and reason are true and reason is the correct explanation of assertion
done
clear
B)
If both assertion and reason are true but reason is not the correct explanation of assertion
done
clear
C)
If assertion is true but reason is false
done
clear
D)
If both assertion and reason are false
done
clear
View Answer play_arrow
question_answer 47) Assertion: Dielectric polarization means formation of positive and negative charges inside the dielectric. Reason: Free electrons are formed in this process.
A)
If both assertion and reason are true and reason is the correct explanation of assertion
done
clear
B)
If both assertion and reason are true but reason is not the correct explanation of assertion
done
clear
C)
If assertion is true but reason is false
done
clear
D)
If both assertion and reason are false
done
clear
View Answer play_arrow
question_answer 48) Assertion: When a magnet is brought near iron nails, only translatory force act on it. Reason: The field due to a magnet is generally uniform.
A)
If both assertion and reason are true and reason is the correct explanation of assertion
done
clear
B)
If both assertion and reason are true but reason is not the correct explanation of assertion
done
clear
C)
If assertion is true but reason is false
done
clear
D)
If both assertion and reason are false
done
clear
View Answer play_arrow
question_answer 49) Assertion: Static crashes are heard on radio, when lightning flash occurs in the sky. Reason: Electromagnetic waves having frequency of radio wave range, interfere with radio waves.
A)
If both assertion and reason are true and reason is the correct explanation of assertion
done
clear
B)
If both assertion and reason are true but reason is not the correct explanation of assertion
done
clear
C)
If assertion is true but reason is false
done
clear
D)
If both assertion and reason are false
done
clear
View Answer play_arrow
question_answer 50) Assertion: The satellites equipped with electronic devices are called active satellites. Reason: Passive satellite works as active satellite.
A)
If both assertion and reason are true and reason is the correct explanation of assertion
done
clear
B)
If both assertion and reason are true but reason is not the correct explanation of assertion
done
clear
C)
If assertion is true but reason is false
done
clear
D)
If both assertion and reason are false
done
clear
View Answer play_arrow
question_answer 51) Maximum entropy will be in which of the following?
A)
Ice
done
clear
B)
Liquid water
done
clear
C)
Snow
done
clear
D)
Water vapors
done
clear
View Answer play_arrow
question_answer 52) What is obtained when chlorine is passed in boiling toluene and product is hydrolyzed?
A)
o-cresol
done
clear
B)
p-cresol
done
clear
C)
2, 4-dihydroxy toluene
done
clear
D)
Benzyl alcohol
done
clear
View Answer play_arrow
question_answer 53) Which of the following has covalent bond?
A)
\[N{{a}_{2}}S\]
done
clear
B)
\[AlC{{l}_{3}}\]
done
clear
C)
\[NaH\]
done
clear
D)
\[MgC{{l}_{2}}\]
done
clear
View Answer play_arrow
question_answer 54) Which of the following acts as an oxidising as well as reducing agent?
A)
\[N{{a}_{2}}O\]
done
clear
B)
\[N{{a}_{2}}{{O}_{2}}\]
done
clear
C)
\[NaN{{O}_{3}}\]
done
clear
D)
\[NaN{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 55) What is the oxidation state of P in \[Ba{{({{H}_{2}}P{{O}_{2}})}_{2}}\].
A)
+ 1
done
clear
B)
+ 2
done
clear
C)
+ 3
done
clear
D)
\[-1\]
done
clear
View Answer play_arrow
question_answer 56) Which of the following molecules has pyramidal shape?
A)
\[PC{{l}_{3}}\]
done
clear
B)
\[S{{O}_{3}}\]
done
clear
C)
\[CO_{3}^{2-}\]
done
clear
D)
\[NO_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 57) Maximum number of hydrogen bonding in \[{{\text{H}}_{\text{2}}}\text{O}\] is :
A)
1
done
clear
B)
2
done
clear
C)
3
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 58) Number of isomers possible for \[{{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\text{O}\]is:
A)
3
done
clear
B)
4
done
clear
C)
5
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 59) \[{{C}_{6}}{{H}_{5}}-CH=CHCHO\xrightarrow{X}\]\[{{C}_{6}}{{H}_{5}}CH=CHC{{H}_{2}}OH\] In the above sequence X can be:
A)
\[{{H}_{2}}/Ni\]
done
clear
B)
\[NaB{{H}_{4}}\]
done
clear
C)
\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}/{{H}^{+}}\]
done
clear
D)
both and
done
clear
View Answer play_arrow
question_answer 60) For a reaction \[{{\text{H}}_{\text{2}}}{{\text{I}}_{\text{2}}}\rightleftharpoons \text{2HI}\]at 721 K, the value of equilibrium constant is 50. If 0.5 mole each of \[{{\text{H}}_{\text{2}}}\]and \[{{\text{I}}_{2}}\] is added to the system, the value of equilibrium constant will be :
A)
40
done
clear
B)
60
done
clear
C)
50
done
clear
D)
30
done
clear
View Answer play_arrow
question_answer 61) Schottky defect generally appears in:
A)
\[\text{NaCl}\]
done
clear
B)
\[\text{KCl}\]
done
clear
C)
\[\text{CsCl}\]
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 62) The ability of a given substance to assume two or more crystalline structure is called:
A)
amorphism
done
clear
B)
isomorphism
done
clear
C)
polymorphism
done
clear
D)
isomerism
done
clear
View Answer play_arrow
question_answer 63) A cricket ball of 0.5 kg is moving with a velocity of 100 m/sec. The wavelength associated with its motion is :
A)
\[1/100\,cm\]
done
clear
B)
\[6.6\times {{10}^{-34}}\,m\]
done
clear
C)
\[1.32\,\times {{10}^{-35}}m\]
done
clear
D)
\[6.6\times {{10}^{-28}}\,m\]
done
clear
View Answer play_arrow
question_answer 64) Which among the following species have the same number of electrons in its outermost as well as penultimate shell?
A)
\[M{{g}^{2+}}\]
done
clear
B)
\[{{\text{O}}^{2-}}\]
done
clear
C)
\[{{F}^{-}}\]
done
clear
D)
\[C{{a}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 65) \[{{}^{\text{o}}}\] of combustion of isobutylene is \[-x\,\text{kJ}\,\text{mo}{{\text{l}}^{-1}}\] The value of \[\Delta {{H}^{o}}\] is:
A)
\[=\Delta {{E}^{o}}\]
done
clear
B)
\[>\Delta {{E}^{o}}\]
done
clear
C)
\[=0\]
done
clear
D)
\[<\Delta {{E}^{o}}\]
done
clear
View Answer play_arrow
question_answer 66) At \[\text{90}{{\,}^{\text{o}}}\text{C,}\] pure water has \[{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{+}}}\] ion concentration of \[{{10}^{-6}}\,\text{mol/}{{\text{L}}^{-1}}.\] The \[{{\text{K}}_{\text{w}}}\] at \[\text{90}{{\,}^{\text{o}}}\text{C}\] is:
A)
\[{{10}^{-6}}\]
done
clear
B)
\[{{10}^{-14}}\]
done
clear
C)
\[{{10}^{-12}}\]
done
clear
D)
\[{{10}^{-8}}\]
done
clear
View Answer play_arrow
question_answer 67) A gas is found to have a formula \[{{[CO]}_{x}}.\] If its vapour density is 70, the value of \[x\] is :
A)
2.5
done
clear
B)
3.0
done
clear
C)
5.0
done
clear
D)
6.0
done
clear
View Answer play_arrow
question_answer 68) Which of the following represents soap?
A)
\[\text{ }\!\!~\!\!\text{ }{{\text{C}}_{\text{17}}}{{\text{H}}_{\text{35}}}\text{COOK}\]
done
clear
B)
\[{{C}_{17}}{{H}_{35}}COOH\]
done
clear
C)
\[{{C}_{15}}{{H}_{31}}COOH\]
done
clear
D)
\[{{({{C}_{17}}{{H}_{35}}COO)}_{2}}Ca\]
done
clear
View Answer play_arrow
question_answer 69) Aspirin is chemically:
A)
methyl benzoate
done
clear
B)
ethyl salicylate
done
clear
C)
ethyl salicylic acid
done
clear
D)
2-acetoxy benzoic acid
done
clear
View Answer play_arrow
question_answer 70) Vitamin \[{{\text{B}}_{\text{6}}}\] is known as:
A)
pyridoxin
done
clear
B)
thiamine
done
clear
C)
tocopherol
done
clear
D)
riboflavin
done
clear
View Answer play_arrow
question_answer 71) Which of the following compounds is found abundantly in nature?
A)
Fructose
done
clear
B)
Starch
done
clear
C)
Glucose
done
clear
D)
Cellulose
done
clear
View Answer play_arrow
question_answer 72) Synthetic polymer which resembles natural rubber is:
A)
neoprene
done
clear
B)
chloroprene
done
clear
C)
glyptal
done
clear
D)
nylon
done
clear
View Answer play_arrow
question_answer 73) Which of the following is not a nitro-derivative?
A)
\[{{\text{C}}_{6}}{{H}_{5}}N{{O}_{2}}\]
done
clear
B)
\[C{{H}_{3}}C{{H}_{2}}ONO\]
done
clear
C)
done
clear
D)
\[{{C}_{6}}{{H}_{4}}(OH)N{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 74) The reduction of which of the following compound would yield secondary amine?
A)
Alkyl nitrile
done
clear
B)
Carbylamine
done
clear
C)
Primary amine
done
clear
D)
Secondary nitro compound
done
clear
View Answer play_arrow
question_answer 75) Which of the aldehyde is most reactive?
A)
\[{{C}_{6}}{{H}_{5}}\text{ }\text{ }CHO\]
done
clear
B)
\[C{{H}_{3}}CHO\]
done
clear
C)
\[HCHO\]
done
clear
D)
All are equally reactive
done
clear
View Answer play_arrow
question_answer 76) Which of the following does not contain ?COOH group?
A)
Aspirin
done
clear
B)
Benzoic acid
done
clear
C)
Picric acid
done
clear
D)
All have ?COOH group
done
clear
View Answer play_arrow
question_answer 77) Which of the following is dihydric alcohol?
A)
Glycerol
done
clear
B)
Ethylene glycol
done
clear
C)
Catechol
done
clear
D)
Resorcinol
done
clear
View Answer play_arrow
question_answer 78) Ethyl alcohol is heated with conc. \[{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\text{.}\] The product formed is :
A)
\[C{{H}_{3}}-\overset{\text{O}}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-O{{C}_{2}}{{H}_{5}}\]
done
clear
B)
\[{{C}_{2}}{{H}_{6}}\]
done
clear
C)
\[{{C}_{2}}{{H}_{4}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 79) In the first order reaction, the concentration of the reactants is reduced to 25% in one hour. The half life period of the reaction is:
A)
2hr
done
clear
B)
4hr
done
clear
C)
1/2 hr
done
clear
D)
1/4 hr
done
clear
View Answer play_arrow
question_answer 80) For a reaction, \[X(g)\xrightarrow{{}}Y(g)+Z(g)\] the half life period is 10 min. In what period of time would the concentration of X be reduced to 10% of original concentration?
A)
20 min
done
clear
B)
33 min
done
clear
C)
15 min
done
clear
D)
25 min
done
clear
View Answer play_arrow
question_answer 81) The molar freezing point constant for water is \[1.86{{\,}^{o}}\text{C/mol}\text{.}\] If 342 g of cane sugar \[({{C}_{12}}{{H}_{22}}{{O}_{11}})\] is dissolved in 1000 g of water, the solution will freeze at:
A)
\[-1.86{{\,}^{o}}C\]
done
clear
B)
\[1.86{{\,}^{o}}C\]
done
clear
C)
\[-3.92{{\,}^{o}}C\]
done
clear
D)
\[2.42{{\,}^{o}}C\]
done
clear
View Answer play_arrow
question_answer 82) The movement of solvent molecules through a semi-permeable membrane is called :
A)
electrolysis
done
clear
B)
electrophoresis
done
clear
C)
osmosis
done
clear
D)
cataphoresis
done
clear
View Answer play_arrow
question_answer 83) Which of the following is a primary halide?
A)
Isopropyl iodide
done
clear
B)
Secondary butyl iodide
done
clear
C)
Tertiary butyl bromide
done
clear
D)
Neo hexyl chloride
done
clear
View Answer play_arrow
question_answer 84) A gas is found to have the formula \[{{\text{(CO)}}_{\text{n}}}\text{.}\] If its vapour density is 56, the value of n will be
A)
7
done
clear
B)
5
done
clear
C)
4
done
clear
D)
3
done
clear
View Answer play_arrow
question_answer 85) Aromatisation of n-heptane by passing over \[(A{{l}_{2}}{{O}_{3}}+C{{r}_{2}}{{O}_{3}})\]catalyst at 773 K gives:
A)
benzene
done
clear
B)
toluene
done
clear
C)
mixture of both
done
clear
D)
heptylene
done
clear
View Answer play_arrow
question_answer 86) \[{{C}_{6}}{{H}_{5}}C{{H}_{3}}\xrightarrow{Cr{{O}_{2}}C{{l}_{2}}}Z\] In the given sequence Z is:
A)
benzaldehyde
done
clear
B)
toluic acid
done
clear
C)
phenyl acetic acid
done
clear
D)
benzoic acid
done
clear
View Answer play_arrow
question_answer 87) Nitroethane can exhibit one of the following kind of isomerism:
A)
metamerism
done
clear
B)
optical activity
done
clear
C)
tautomerism
done
clear
D)
position isomerism
done
clear
View Answer play_arrow
question_answer 88) A compound has three chiral carbon atoms. The number of possible optical isomers it can have :
A)
3
done
clear
B)
2
done
clear
C)
8
done
clear
D)
4
done
clear
View Answer play_arrow
question_answer 89) \[4{{K}_{2}}C{{r}_{2}}{{O}_{7}}\xrightarrow{\text{heat}}4{{K}_{2}}Cr{{O}_{4}}+3{{O}_{2}}+X.\] In the above reaction X is:
A)
\[Cr{{O}_{3}}\]
done
clear
B)
\[C{{r}_{2}}{{O}_{7}}\]
done
clear
C)
\[C{{r}_{2}}{{O}_{3}}\]
done
clear
D)
\[Cr{{O}_{5}}\]
done
clear
View Answer play_arrow
question_answer 90) The co-ordination number and oxidation number of X in the following compound \[[X(S{{O}_{4}}){{(N{{H}_{3}})}_{5}}]Cl\]will be:
A)
10 and 3
done
clear
B)
2 and 6
done
clear
C)
6 and 3
done
clear
D)
6 and 4
done
clear
View Answer play_arrow
question_answer 91) In the electrolysis of water, one faraday of electrical energy would evolve :
A)
one mole of oxygen
done
clear
B)
one gram atom of oxygen
done
clear
C)
8 g of oxygen
done
clear
D)
22.4 Lot oxygen
done
clear
View Answer play_arrow
question_answer 92) In which of these processes platinum is used as a catalyst?
A)
Oxidation of ammonia to form \[\text{HN}{{\text{O}}_{\text{3}}}\]
done
clear
B)
Hardening of oils
done
clear
C)
Production of synthetic rubber
done
clear
D)
Synthesis of methanol
done
clear
View Answer play_arrow
question_answer 93) If the half-life of an isotope X is 10 years, its decay constant is :
A)
\[6.932y{{r}^{-1}}\]
done
clear
B)
\[0.6932\text{ }y{{r}^{-1}}\]
done
clear
C)
\[0.06932\text{ }y{{r}^{-1}}\]
done
clear
D)
\[0.006932\text{ }y{{r}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 94) On strong heating sodium bicarbonate changes into:
A)
sodium monoxide
done
clear
B)
sodium hydroxide
done
clear
C)
sodium carbonate
done
clear
D)
sodium peroxide
done
clear
View Answer play_arrow
question_answer 95) Which of the following is not an ore of magnesium?
A)
Camallite
done
clear
B)
Magnesite
done
clear
C)
Dolomite
done
clear
D)
Gypsum
done
clear
View Answer play_arrow
question_answer 96) Aluminium reacts with caustic soda to form:
A)
aluminium hydroxide
done
clear
B)
aluminium oxide
done
clear
C)
sodium meta-aluminate
done
clear
D)
sodium tetra aluminate
done
clear
View Answer play_arrow
question_answer 97) In laboratory burners, we use:
A)
producer gas
done
clear
B)
oil gas
done
clear
C)
gobar gas
done
clear
D)
coal gas
done
clear
View Answer play_arrow
question_answer 98) Iron is dropped in dil. \[\text{HN}{{\text{O}}_{\text{3}}}\text{,}\]it gives:
A)
ferric nitrate
done
clear
B)
ferric nitrate and \[\text{N}{{\text{O}}_{2}}\]
done
clear
C)
ferrous nitrate and ammonium nitrate
done
clear
D)
ferrous nitrate and nitric oxide
done
clear
View Answer play_arrow
question_answer 99) When tin is treated with concentrated nitric acid:
A)
it is converted into stannous nitrate
done
clear
B)
it is converted into stannic nitrate
done
clear
C)
it is converted into metastannic acid
done
clear
D)
it becomes passive
done
clear
View Answer play_arrow
question_answer 100) The chief impurity present in red bauxite is:
A)
\[Si{{O}_{2}}\]
done
clear
B)
\[F{{e}_{2}}{{O}_{3}}\]
done
clear
C)
\[{{K}_{2}}S{{O}_{4}}\]
done
clear
D)
\[NaF\]
done
clear
View Answer play_arrow
question_answer 101) Endosperm which is the common nutritive tissue for the developing embryo in angiosperm develops from:
A)
micropylar polar nucleus
done
clear
B)
chalazal polar nucleus
done
clear
C)
secondary nucleus
done
clear
D)
zygote
done
clear
View Answer play_arrow
question_answer 102) Bryophytes are dependent on water because:
A)
archegonium has to remain filled with water for fertilization
done
clear
B)
water is essential for fertilization for their homosporous water
done
clear
C)
water is essential for their vegetative propagation
done
clear
D)
the sperms can easily reach upto egg in the archegonium
done
clear
View Answer play_arrow
question_answer 103) The protective device over the developing sporophyte is shoot calyptra in:
A)
Frullania
done
clear
B)
Sphagnum
done
clear
C)
Anthoceros
done
clear
D)
Pellia
done
clear
View Answer play_arrow
question_answer 104) Which of the following is finally reabsorbed in distal convoluted tubule?
A)
Calcium
done
clear
B)
Potassium
done
clear
C)
Bicarbonate
done
clear
D)
Water
done
clear
View Answer play_arrow
question_answer 105) Which one of the following is metabolic waste of protein metabolism ?
A)
Urea, ammonia and \[C{{O}_{2}}\]
done
clear
B)
Urea, ammonia and creatinine
done
clear
C)
Urea, ammonia and alanine
done
clear
D)
Urea, nitrogen and \[{{O}_{2}}\]
done
clear
View Answer play_arrow
question_answer 106) What is true of urea biosynthesis ?
A)
Uric acid is starting point
done
clear
B)
Urea is synthesized in lysosomes
done
clear
C)
Urea cycle enzymes are located inside mitochondria
done
clear
D)
Urea is synthesized in kidney
done
clear
View Answer play_arrow
question_answer 107) The posterior vena cava or interior vena cava:
A)
divides into the hepatic portal veins
done
clear
B)
opens into the left auricle
done
clear
C)
commences at the kidney
done
clear
D)
begins at the hind end of abdomen
done
clear
View Answer play_arrow
question_answer 108) Layers of cells that secrete enamel of tooth is:
A)
osteoblast
done
clear
B)
ameloblast
done
clear
C)
odontoblast
done
clear
D)
dontoblast
done
clear
View Answer play_arrow
question_answer 109) Which one of the following is the correct sequence in the development of frog ?
A)
Clevage \[\to \] Morula \[\to \] Blastula\[\to \] Gastrula
done
clear
B)
Gastrula \[\to \] Neurula\[\to \] Blastula\[\to \] Morula
done
clear
C)
Morula \[\to \] Neurula \[\to \] Gastrula \[\to \] Blastula
done
clear
D)
lastula \[\to \] Gastrula \[\to \]Neurula \[\to \] Morula
done
clear
View Answer play_arrow
question_answer 110) During DNA replication, the strands separated by:
A)
DNA polymerase
done
clear
B)
topoisomerase
done
clear
C)
unwindase/helecase
done
clear
D)
gyrase
done
clear
View Answer play_arrow
question_answer 111) Presence of recessive trait is 16%. The frequency of dominant allele in population is:
A)
0.6
done
clear
B)
0.32
done
clear
C)
0.84
done
clear
D)
0.92
done
clear
View Answer play_arrow
question_answer 112) Frequency of an autosomal lethal gene is 0 4. The frequency of carrier in a population or 200 indivedual is:
A)
72
done
clear
B)
96
done
clear
C)
104
done
clear
D)
36
done
clear
View Answer play_arrow
question_answer 113) According to genic balance theory of Bridges X/A = 1.5 will make the individual:
A)
male
done
clear
B)
female
done
clear
C)
intersex
done
clear
D)
super female
done
clear
View Answer play_arrow
question_answer 114) Cuscuta which climbs up the support with help of loop formation around the support. This type of movement is called:
A)
nutation
done
clear
B)
nastic movement
done
clear
C)
hygroscopic movement
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 115) Which of the following is a cultural characteristics of micro-organism?
A)
Nutritional requirement for growth
done
clear
B)
Cell-shape
done
clear
C)
Cell-size
done
clear
D)
Cell structure
done
clear
View Answer play_arrow
question_answer 116) The cells of the bacterium Streptococcus remain arranged in the form of:
A)
irregular cluster
done
clear
B)
chain
done
clear
C)
plate
done
clear
D)
cube
done
clear
View Answer play_arrow
question_answer 117) Interferon suppresses the pathogenic activity of:
A)
viruses
done
clear
B)
bacteria
done
clear
C)
protozoa
done
clear
D)
all of these
done
clear
View Answer play_arrow
question_answer 118) A virus consist of:
A)
lipid coat (capsid), genes and ribosomes
done
clear
B)
cell membrane and chromosome
done
clear
C)
protein coat genes and mitochondria
done
clear
D)
protein coat and nucleic acid molecules
done
clear
View Answer play_arrow
question_answer 119) Many species of marine bacteria characteristically grow while attached to a solid suaface and are called as:
A)
periphytes
done
clear
B)
epibacteria
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 120) Which of the following is the speciality of \[\phi \times 174\,\] and M - 13 type of viruses?
A)
Single-stranded DNA
done
clear
B)
Single-stranded RNA
done
clear
C)
Double- stranded DNA
done
clear
D)
Double-stranded RNA
done
clear
View Answer play_arrow
question_answer 121) The site of respiration in bacteria is:
A)
microsome
done
clear
B)
mesosomes
done
clear
C)
episome
done
clear
D)
ribosome
done
clear
View Answer play_arrow
question_answer 122) Choose the correct sequence during the formation of chemicals on early earth:
A)
ammonia, water, nucleic acid and proteins
done
clear
B)
ammonia, proteins, carbohydrates and nucleic acid
done
clear
C)
ammonia, nucleic acid, protein and carbohydrate
done
clear
D)
protion, carbohydrate, water and nucleic acid
done
clear
View Answer play_arrow
question_answer 123) Mutation theory to explain mechanism of evolution were given by Hugo de Vries and he experimented on :
A)
garden pea
done
clear
B)
fruit fly
done
clear
C)
china rose
done
clear
D)
evening primrose
done
clear
View Answer play_arrow
question_answer 124) Replica plating experiment was conducted by :
A)
Joshual Lederberg
done
clear
B)
R.A. Fischer
done
clear
C)
Esther Lederberg
done
clear
D)
Both (a) and (c)
done
clear
View Answer play_arrow
question_answer 125) Organism present at different places without any traces in between show :
A)
speciation
done
clear
B)
discontinuous distribution
done
clear
C)
punctiued Equilibuiun
done
clear
D)
migration
done
clear
View Answer play_arrow
question_answer 126) Who attempted to solve the mechanism of organic evolution for the first time ?
A)
Haeckal
done
clear
B)
Hugo de Vries
done
clear
C)
Lamark
done
clear
D)
Darwin
done
clear
View Answer play_arrow
question_answer 127) Some of the important evidences of organic evolution are:
A)
occurence of homologous and vestigial organs
done
clear
B)
occurence of analogous and vestigial organs
done
clear
C)
occurence of homologous and analogous organs
done
clear
D)
occurence of analogous organs only
done
clear
View Answer play_arrow
question_answer 128) Fossils of Archaeopteryx reveals that:
A)
reptiles were most evolved during Jurassic
done
clear
B)
toothed birds originated from flying reptiles during Jurassic
done
clear
C)
toothed birds originated during Jurassic
done
clear
D)
toothed birds gave rise to primitive mammals
done
clear
View Answer play_arrow
question_answer 129) Wasteland reclamation is best brought by:
A)
Prosopis
done
clear
B)
Acacia
done
clear
C)
Leucaena
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 130) The average net primany productivity of which of these community is highest:
A)
tropical rain forest
done
clear
B)
temperate deciduous forest
done
clear
C)
rocky beaches and sandy beaches
done
clear
D)
tundra
done
clear
View Answer play_arrow
question_answer 131) An estuary acts as a nutrient trap because of the:
A)
action of rivers and tides
done
clear
B)
depth at which photosynthesis can occur
done
clear
C)
amount of rainfall received
done
clear
D)
height of water table
done
clear
View Answer play_arrow
question_answer 132) The force operating in an ecosystem which opposes the unchecked growth of population is:
A)
mortality
done
clear
B)
biotic control
done
clear
C)
environmental resistance
done
clear
D)
fecundity
done
clear
View Answer play_arrow
question_answer 133) Out of the following given ecosystem which one is most recently discovered ecosystem :
A)
Tundra
done
clear
B)
Crater
done
clear
C)
Ice bug
done
clear
D)
Vents
done
clear
View Answer play_arrow
question_answer 134) Chapparal are evergreen broad leaved fire resistant drought evading scrub forests (Sclerophyllous plants with hard thick leaves) found in mediterenean area, Africa and Australia. These are characterized by:
A)
heavy rain in summer and winter dry
done
clear
B)
rain throughout year and no bush fire
done
clear
C)
limited rain in winter, summer dry and bush fire
done
clear
D)
little rain in summer and bush fire
done
clear
View Answer play_arrow
question_answer 135) In desert grasslands, which type of animals are relatively more abundant ?
A)
Diurnal
done
clear
B)
Nocturnal
done
clear
C)
Arboreal
done
clear
D)
Fossorial
done
clear
View Answer play_arrow
question_answer 136) If 20 k cal energy is available at producer level then how much energy will be transfered to the lion in the food chain-producer \[\to \] deer \[\to \] lion ?
A)
0.2 J
done
clear
B)
0.02 J
done
clear
C)
0.002 J
done
clear
D)
2 J
done
clear
View Answer play_arrow
question_answer 137) Which of the following is pollutant as well as protectant?
A)
\[C{{O}_{2}}\]
done
clear
B)
Ozone
done
clear
C)
CFC
done
clear
D)
PAN
done
clear
View Answer play_arrow
question_answer 138) If the dicot stem is stained for starch. The most intense colouration would develop in:
A)
epiblema
done
clear
B)
phloem
done
clear
C)
endodermis
done
clear
D)
pith
done
clear
View Answer play_arrow
question_answer 139) The layer of parenchyma surrounding a vascular bundle is known as:
A)
bundle sheath
done
clear
B)
bundle shield
done
clear
C)
bundle parenchyma
done
clear
D)
bundle fibre
done
clear
View Answer play_arrow
question_answer 140) Although the seeds are rich in auxin glucosyl esters, still they do not exhibit any physiological change like growth. The reason is:
A)
they are prime examples of bound auxins
done
clear
B)
they are example of bound auxins that are inactive untill IAA is released by enzymolysis
done
clear
C)
that they are examples of free auxins
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 141) The plant hormone used for inducing morphogeneces in plant tissue culture is:
A)
cytokinin
done
clear
B)
abscisic acid
done
clear
C)
gibberellin
done
clear
D)
ethylene
done
clear
View Answer play_arrow
question_answer 142) The phytochemical ?quercetin? is found in which of the following ?
A)
grapes
done
clear
B)
apples
done
clear
C)
guava
done
clear
D)
lemon
done
clear
View Answer play_arrow
question_answer 143) Plants flower in response to a certain ratio of light to dark in a day, which reflects seasonal changes. This response is known as:
A)
verbalization
done
clear
B)
photoperiodism
done
clear
C)
photorespiration
done
clear
D)
photosynthesis
done
clear
View Answer play_arrow
question_answer 144) A sleep movement is a mastic response that occurs daily in response to :
A)
dark
done
clear
B)
light
done
clear
C)
both (a) and (b)
done
clear
D)
none of these
done
clear
View Answer play_arrow
question_answer 145) Sedoheptulose 7-phosphate reacts with 3-phosphoglyceraldehyde during dark reaction to form:
A)
ribulose 5-phosphate
done
clear
B)
xylulose 5-phosphate
done
clear
C)
ribose 5-phosphate
done
clear
D)
both (b) and (c)
done
clear
View Answer play_arrow
question_answer 146) Photosynthesis in CAM-plants is minimal because of the limited amount of:
A)
\[C{{O}_{2}}\] fixed at night
done
clear
B)
\[{{O}_{2}}\] fixed at night
done
clear
C)
\[{{O}_{2}}\] fixed in day light
done
clear
D)
\[C{{O}_{2}}\] fixed in day light
done
clear
View Answer play_arrow
question_answer 147) Site of Krebs cycle and ATP synthesis in bacteria prokaryotes is:
A)
cell wall
done
clear
B)
mitochondria
done
clear
C)
plasma membrane
done
clear
D)
nucleoid
done
clear
View Answer play_arrow
question_answer 148) Poisons like cyanides inhibit \[N{{a}^{+}}\] effux and \[{{K}^{+}}\] influx. The effect is reversed by injection of ATP indicating that:
A)
\[N{{a}^{+}}\to {{K}^{+}}\] pump operates in all cells
done
clear
B)
ATP is carrier protein
done
clear
C)
Energy for \[N{{a}^{+}}-{{K}^{+}}\] pump comes from ATP
done
clear
D)
ATP is hydrolysed by ATPase to release energy
done
clear
View Answer play_arrow
question_answer 149) Which of these acts as a connecting link between respiration and protein synthesis ?
A)
Acetyl co-A
done
clear
B)
\[\alpha \]-ketoglutaric acid
done
clear
C)
Pyruvic acid
done
clear
D)
PGAL
done
clear
View Answer play_arrow
question_answer 150) Substrate phosphorylation occur during:
A)
fumaric acid\[\to \] malic acid
done
clear
B)
oxalosuccinic acid\[\to \] \[\alpha \]-ketoglutaric acid
done
clear
C)
succinic acid\[\to \] fumaric acid
done
clear
D)
\[\alpha \]-ketoglutaric acid\[\to \] succinic acid
done
clear
View Answer play_arrow
question_answer 151) Most of the biological energy is supplied by mitrochondria through:
A)
breaking of proteins
done
clear
B)
reduction of NADP
done
clear
C)
breaking of sugar
done
clear
D)
Oxidizing TCA substrate
done
clear
View Answer play_arrow
question_answer 152) Fixing up of the blastocyst in the wall of the uterus is known as:
A)
placentation
done
clear
B)
fertilization
done
clear
C)
implantation
done
clear
D)
impregnation
done
clear
View Answer play_arrow
question_answer 153) Absence of sexual union and failure of meiosis is known as:
A)
anisospory
done
clear
B)
apomixes
done
clear
C)
amphimixis
done
clear
D)
anisogamety
done
clear
View Answer play_arrow
question_answer 154) Teolme theory of Zimmerman (1930) applies only to:
A)
bryophytes
done
clear
B)
pteridophytes
done
clear
C)
tracheophytes
done
clear
D)
all plants
done
clear
View Answer play_arrow
question_answer 155) Heterospory and seed habit are exhibited by a non-flowering plant which also possess:
A)
bract
done
clear
B)
ligule
done
clear
C)
petiole
done
clear
D)
spathe
done
clear
View Answer play_arrow
question_answer 156) The term "allelomorphic? implies:
A)
any two characters
done
clear
B)
a pair of contrasting character
done
clear
C)
sex-linked characters
done
clear
D)
a pair of non-contrasting character
done
clear
View Answer play_arrow
question_answer 157) If a homozygous red flowered plant is crossed with a homozygous white flowered plant, the offsprings will be:
A)
all red flowered
done
clear
B)
all white flowered
done
clear
C)
half red flowered
done
clear
D)
half white flowered
done
clear
View Answer play_arrow
question_answer 158) How many pair of contrasting characters were chosen by Mendel for his study with garden pea?
A)
3
done
clear
B)
7
done
clear
C)
5
done
clear
D)
14
done
clear
View Answer play_arrow
question_answer 159) In DNA the two chains of double helix run opposite to each other. This head to tail arrangement is called :
A)
anti parallelism
done
clear
B)
semi conservatism
done
clear
C)
autoconservatism
done
clear
D)
alternation
done
clear
View Answer play_arrow
question_answer 160) If the sequence of bases in the DNA is TAGC, then the sequence of bases in m-RNA will be :
A)
ATCG
done
clear
B)
AUCG
done
clear
C)
TAGC
done
clear
D)
TACG
done
clear
View Answer play_arrow
question_answer 161) In DNA when AGCT occurs, their association is as per which of the following pair ?
A)
AC-GT
done
clear
B)
AG-CT
done
clear
C)
AT-GC
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 162) What would be the colour of flowers in \[{{F}_{1}}\] progeny as a result of cross between homozygous red and homozygous white flower snapdragon?
A)
Red
done
clear
B)
White
done
clear
C)
Both (a) and (b)
done
clear
D)
Pink
done
clear
View Answer play_arrow
question_answer 163) Drosophila melanogaster has:
A)
2 pair of autosomes and one pair of sex chromosome
done
clear
B)
3 pair of autosomes and one pair of sex chromosomes
done
clear
C)
1 pair of autosomes and 3 pair of sex chromosomes
done
clear
D)
2 pair of autosomes and 2 pair of sex chromosomes
done
clear
View Answer play_arrow
question_answer 164) DNA replication is:
A)
conservative
done
clear
B)
semi-conservative
done
clear
C)
non-conservative
done
clear
D)
complex
done
clear
View Answer play_arrow
question_answer 165) Genetic engineering is possible because:
A)
the phenomenon of transduction in bacteria is well understood
done
clear
B)
restriction endonuclease purified from bacteria can be used in vitro
done
clear
C)
we can see DNA at specific setes by endonucleases like DNAase I
done
clear
D)
we can see DNA by electron microscope
done
clear
View Answer play_arrow
question_answer 166) The first gentically engineered human insulin was launched in the year :
A)
1975
done
clear
B)
1993
done
clear
C)
1990
done
clear
D)
1983
done
clear
View Answer play_arrow
question_answer 167) In which of the following the sporophyte is indeterminate in growth?
A)
Riccia
done
clear
B)
Marchantia
done
clear
C)
Anthoceros
done
clear
D)
Funaria
done
clear
View Answer play_arrow
question_answer 168) In Sphagnum, the gametophyte structure compensating for the absence of seta is known as :
A)
columella
done
clear
B)
elatrophore
done
clear
C)
sporangiophore
done
clear
D)
pseudopodium
done
clear
View Answer play_arrow
question_answer 169) Which one of the following is a characteristic of dicotyledons ?
A)
Large xylem in roots and multicellular hairs
done
clear
B)
Fibrous root system and scattered vascular bundle in stem
done
clear
C)
Cotyledons are 2 and terminal in position
done
clear
D)
Floral parts in multiples of 4 or 5 and stem has vascular bundles in a ring
done
clear
View Answer play_arrow
question_answer 170) Which statement is wrong for Cuscuta ?
A)
It is a holoparasite of stem and sends its haustoria into xylem and phloem of host
done
clear
B)
It is a dicot without any cotyledons
done
clear
C)
If it grows on a Short Day Plant (SDP) it becomes SDP and when it grows on a Long Day Plant (LDP) it becomes LDP
done
clear
D)
Its haustorial roots arise from radicle
done
clear
View Answer play_arrow
question_answer 171) Cotton fibres which are obtained from Gossypium are:
A)
surface fibre
done
clear
B)
soft fibre
done
clear
C)
hard fibre
done
clear
D)
bast fibre
done
clear
View Answer play_arrow
question_answer 172) Semul fibre which are used as filling fibres are obtained from:
A)
Bombyx ceiba
done
clear
B)
Calotropis procera
done
clear
C)
Hibiscus elatus
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 173) Biogenesis of chloroplast and mitochondria is:
A)
de novo
done
clear
B)
from ER/nuclear membrane
done
clear
C)
from persisting organelle
done
clear
D)
symbiotic origin
done
clear
View Answer play_arrow
question_answer 174) Agranal chloroplast is common in:
A)
\[{{C}_{3}}\] plants
done
clear
B)
Hydrophytes
done
clear
C)
\[{{C}_{4}}\] plants
done
clear
D)
Succulents
done
clear
View Answer play_arrow
question_answer 175) A pigment in cartenoid is found in bacteria and fungi, it is:
A)
fuxocanthin
done
clear
B)
lycopene
done
clear
C)
capsanthin
done
clear
D)
xanthophy
done
clear
View Answer play_arrow
question_answer 176) The inner membrane of mitochondria bears folding/finger like projections called cristae, these cristae:
A)
increases thickness of wall
done
clear
B)
increase surface area
done
clear
C)
increase ATP supply
done
clear
D)
keep external substance away
done
clear
View Answer play_arrow
question_answer 177) What is mioplast?
A)
Another name of mitochondria
done
clear
B)
Membraneless mitochondria
done
clear
C)
Mitochondria without outer membrane
done
clear
D)
Mitochondria without inner membrane
done
clear
View Answer play_arrow
question_answer 178) In germinating oil seeds p-oxidation of free fatty acids/fat digestion takes place in:
A)
glyoxysomes
done
clear
B)
cytosol
done
clear
C)
mitochondria
done
clear
D)
sphaerosomes
done
clear
View Answer play_arrow
question_answer 179) Lysosomes were discovered by de Duve accidently. Who gave the term lysosome and examined under electron microscope?
A)
Fitz James
done
clear
B)
Novikoff
done
clear
C)
Palade
done
clear
D)
Robertson
done
clear
View Answer play_arrow
question_answer 180) The organelles which occur external to cell membrane but internal to cell wall are:
A)
peroxisomes
done
clear
B)
glyoxysomes
done
clear
C)
sphaerosomes
done
clear
D)
lomasomes
done
clear
View Answer play_arrow
question_answer 181) The filaments present in cilia and flagella are composed of:
A)
microtubules
done
clear
B)
microfilaments
done
clear
C)
microfibrils
done
clear
D)
microvilli
done
clear
View Answer play_arrow
question_answer 182) 0 + 9 microfibrillar structure is found in:
A)
basal body
done
clear
B)
blepharoplast
done
clear
C)
centriole
done
clear
D)
all correct
done
clear
View Answer play_arrow
question_answer 183) Sleeping sickness is cause by:
A)
Trypanosoma gambience
done
clear
B)
Plasmodium vivax
done
clear
C)
Leishmania donovani
done
clear
D)
Trypanosoma cruzi
done
clear
View Answer play_arrow
question_answer 184) In which of the following sub-classes of Reptile the skull has a social roof?
A)
Anapsida
done
clear
B)
Diapsida
done
clear
C)
Segnapsida
done
clear
D)
Parapsida
done
clear
View Answer play_arrow
question_answer 185) Cockroach, housefly and mosquitoes are insects because they have:
A)
chitinous exoskeleton and body divided into head and cephalo thorax
done
clear
B)
six legs, ocellie and body divided into head, thorax and abdomen
done
clear
C)
segmented body with jointed feet and chitinous exoskeleton
done
clear
D)
three pairs of legs, one pair of antennae and flame cells
done
clear
View Answer play_arrow
question_answer 186) Diagnostic character of Echinodermata is the presence of:
A)
water vascular system
done
clear
B)
spiny skin
done
clear
C)
pedicellariae
done
clear
D)
all of the above
done
clear
View Answer play_arrow
question_answer 187) Which one of the following classes has the maximum economic importance?
A)
Gastropoda
done
clear
B)
Myriapoda
done
clear
C)
Pelecypoda
done
clear
D)
Cephalopoda
done
clear
View Answer play_arrow
question_answer 188) Any one of the following set of characters is definetely found at some stage in the life cycle of a chordate:
A)
mammary glands, hair, gill cleft
done
clear
B)
notochord, gill-cleft, dorsal tubular nervous system
done
clear
C)
notochord, vertebral column, gill-cleft
done
clear
D)
dorsal tubular nervous system, vertebral column, scales
done
clear
View Answer play_arrow
question_answer 189) During preservation in the frozen state i.e., freeze preservation or cryopreservation, the germplasm is frozen in:
A)
liquid hydrochloric acid
done
clear
B)
liquid nitrogen
done
clear
C)
liquid oxygen
done
clear
D)
liquid hydrogen
done
clear
View Answer play_arrow
question_answer 190) A bacterium which fixes atmospheric nitrogen in non-legummous plant is:
A)
Azotobacter
done
clear
B)
Rhizopus
done
clear
C)
Azospirillum
done
clear
D)
E. coli
done
clear
View Answer play_arrow
question_answer 191) Which one of the following likely to accumulate in a dangerous proportion in the blood of a person where kidney is not working properly?
A)
Lysine
done
clear
B)
Ammonia
done
clear
C)
Sodium chloride
done
clear
D)
Urea
done
clear
View Answer play_arrow
question_answer 192) Total number of bones found in human skull is:
A)
22
done
clear
B)
29
done
clear
C)
35
done
clear
D)
72
done
clear
View Answer play_arrow
question_answer 193) In mammals, the lower jaw is made of:
A)
madulla
done
clear
B)
dentary
done
clear
C)
nandible
done
clear
D)
ethmoid
done
clear
View Answer play_arrow
question_answer 194) Name the part of body which have single pair of bone :
A)
pelvic girdle
done
clear
B)
external ear
done
clear
C)
wrist
done
clear
D)
lower jaw
done
clear
View Answer play_arrow
question_answer 195) Segregation of Mendelian factors (no linkage no crossing over) occurs during :
A)
anaphase I
done
clear
B)
anaphase II
done
clear
C)
diplotene
done
clear
D)
metaphase I
done
clear
View Answer play_arrow
question_answer 196) Haemophilic man marries a carrier woman. Percentage of daughter becoming haemophilic shall be:
A)
25%
done
clear
B)
50%
done
clear
C)
75%
done
clear
D)
100%
done
clear
View Answer play_arrow
question_answer 197) Difference between lymph and blood is that lymph contains :
A)
more RBC less WBC
done
clear
B)
less RBC more WBC
done
clear
C)
no RBC less WBC
done
clear
D)
no RBC more WBC
done
clear
View Answer play_arrow
question_answer 198) A mature human erythrocyte has the typical characteristic of:
A)
a eukaryotic cell
done
clear
B)
a prokaryotic cell
done
clear
C)
both (a) and (b)
done
clear
D)
none of the above
done
clear
View Answer play_arrow
question_answer 199) pH of blood in arteries and veins is:
A)
higher in arteries and lower in veins
done
clear
B)
higher in veins and lower in arteries
done
clear
C)
same
done
clear
D)
variable in both
done
clear
View Answer play_arrow
question_answer 200) An \[R{{h}^{-}}\] individual recives \[R{{h}^{+}}\] blood. The receipent becomes:
A)
sterile
done
clear
B)
dead
done
clear
C)
no reaction
done
clear
D)
iso-immunised
done
clear
View Answer play_arrow