question_answer 1) Equation of plane wave is given by \[4\sin \frac{\pi }{4}\left[ 2t+\frac{x}{8} \right].\]The phase difference at any given instant of two particles 16 cm apart is
A)
\[{{60}^{o}}\]
done
clear
B)
\[{{90}^{o}}\]
done
clear
C)
\[30{}^\circ \]
done
clear
D)
\[{{120}^{o}}\]
done
clear
View Answer play_arrow
question_answer 2) The angle between the electric lines of force and an equipotential surface
A)
\[{{45}^{o}}\]
done
clear
B)
\[{{90}^{o}}\]
done
clear
C)
\[{{0}^{o}}\]
done
clear
D)
\[{{180}^{o}}\]
done
clear
View Answer play_arrow
question_answer 3) A condenser of capacity \[50\,\mu F\]is charged to 10 V. Its energy is equal to
A)
\[2.5\times {{10}^{-3}}J\]
done
clear
B)
\[2.5\times {{10}^{-4}}J\]
done
clear
C)
\[2.5\times {{10}^{-2}}J\]
done
clear
D)
\[1.25\times {{10}^{-8}}J\]
done
clear
View Answer play_arrow
question_answer 4) Two wires having resistance R and 2R are connected in parallel, the ratio of heat generated in 2R and R is
A)
1:2
done
clear
B)
2 :2
done
clear
C)
1:4
done
clear
D)
4:2
done
clear
View Answer play_arrow
question_answer 5) In a nuclear fission, \[0.1%\]mass is converted into energy. The energy released by the fission of 1 kg mass will be
A)
\[9\times {{10}^{19}}\,J\]
done
clear
B)
\[9\times {{10}^{17}}\,J\]
done
clear
C)
\[9\times {{10}^{16}}\,J\]
done
clear
D)
\[9\times {{10}^{13}}\,J\]
done
clear
View Answer play_arrow
question_answer 6) The energy of hydrogen atom in the nth orbit is \[{{E}_{n,}}\]then the energy in the nth orbit of single ionised helium atom is
A)
\[\frac{{{E}_{n}}}{2}\]
done
clear
B)
\[2{{E}_{n}}\]
done
clear
C)
\[4{{E}_{n}}\]
done
clear
D)
\[\frac{{{E}_{n}}}{4}\]
done
clear
View Answer play_arrow
question_answer 7) Suppose, 1 mg of radioactive substance is taken initially. After 2 h, it is found that 0.25 mg of the substance is left behind. The mean life of the substance is
A)
\[\frac{1}{0.693}h\]
done
clear
B)
\[0.693\times 2h\]
done
clear
C)
\[0.693\times \frac{1}{4}h\]
done
clear
D)
\[0.693\times 8h\]
done
clear
View Answer play_arrow
question_answer 8) Pure silicon at 300 K has equal electrons \[{{n}_{e}}\] and holes \[{{n}_{h}}\] concentration of 1.5 \[1.5\times {{10}^{16}}/{{m}^{3}}.\] Doping by indium increases number of holes\[{{n}_{h}}\]to \[4.5\times {{10}^{22}}/{{m}^{3}},\] then \[{{n}_{e}}\]doped in silicon will be
A)
\[3.0\times {{10}^{-19}}/{{m}^{3}}\]
done
clear
B)
\[5\times {{10}^{9}}/{{m}^{3}}\]
done
clear
C)
\[4.5\times {{10}^{22}}/{{m}^{3}}\]
done
clear
D)
\[1.5\times {{10}^{16}}/{{m}^{3}}\]
done
clear
View Answer play_arrow
question_answer 9) In an atom bomb, the reaction which occurs is
A)
thermo nuclear
done
clear
B)
uncontrolled fission
done
clear
C)
controlled fission
done
clear
D)
fusion
done
clear
View Answer play_arrow
question_answer 10) If the coefficient of static friction between the types and road is 0.5. What is the shortest distance in which an automobile can be stopped when travelling at 72 km/h?
A)
50 m
done
clear
B)
60 m
done
clear
C)
40.8m
done
clear
D)
80.16m
done
clear
View Answer play_arrow
question_answer 11)
What should be the minimum value of refractive index of the material of the prism for the reflection to take place as shown in the figure?
A)
1.7
done
clear
B)
1.4
done
clear
C)
1.2
done
clear
D)
2.7
done
clear
View Answer play_arrow
question_answer 12) An L- C circuit is in the state of resonance. If \[C=0.1\,\mu F\]and \[L=0.25\,H,\]neglecting ohmic resistance of circuit. What is the frequency of oscillations?
A)
1007 Hz
done
clear
B)
100 Hz
done
clear
C)
109 Hz
done
clear
D)
500 Hz
done
clear
View Answer play_arrow
question_answer 13) A body, thrown upwards with some velocity reaches the maximum height of 50 m. Another body with double the mass thrown up with double the initial velocity will reach a maximum height of
A)
100 m
done
clear
B)
200 m
done
clear
C)
300 m
done
clear
D)
400 m
done
clear
View Answer play_arrow
question_answer 14) A 100 m long train is moving with a uniform velocity of 45 km/h. The time taken by the train to cross a bridge of length 1 km is
A)
58 s
done
clear
B)
68 s
done
clear
C)
78 s
done
clear
D)
88 s
done
clear
View Answer play_arrow
question_answer 15) Earth is revolving around the sun. If the distance of the earth from the sun is reduced to 1/4th of the present distance, then the length of present day will be reduced by
A)
1/4
done
clear
B)
1/2
done
clear
C)
1/8
done
clear
D)
1/6
done
clear
View Answer play_arrow
question_answer 16) Two rods of same material have same length and area. The heat \[\Delta Q\]flows through them for 12 min when they are joint side by side. If now both the rods are joined in parallel, then the same amount of heat \[\Delta Q\]will flow in
A)
24 min
done
clear
B)
3 min
done
clear
C)
12 min
done
clear
D)
6 min
done
clear
View Answer play_arrow
question_answer 17) There are 26 tuning forks arranged in the decreasing order of their frequencies. Each tuning fork gives 3 beats with the next. The first one is octave of the last. What is he frequency of 18th tuning fork?
A)
100 Hz
done
clear
B)
\[99\,Hz\]
done
clear
C)
\[96\,Hz\]
done
clear
D)
\[103{{\,}^{Pz}}\]
done
clear
View Answer play_arrow
question_answer 18) Who discovered X-rays?
A)
Rontgen
done
clear
B)
Madam curie
done
clear
C)
Rutherford
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 19)
A body of mass 2 kg has an initial velocity of 3 m/s along OE and it is subjected to a force of 4 N in a direction perpendicular to OE. The distance of body from O after 4 s will be
A)
12 m
done
clear
B)
20 m
done
clear
C)
8 m
done
clear
D)
48 m
done
clear
View Answer play_arrow
question_answer 20) A synchronous relay satellite reflects TV signals and transmits TV programme from one part of the world to the other because its
A)
period of revolution is greate then the period of rotation of the earth about its axis
done
clear
B)
period of revolution is less than the per od of rotation of the earth about its axis
done
clear
C)
period of revolution is equal to the period of rotation of the earth about its axis
done
clear
D)
mass is less than the mass of earn
done
clear
View Answer play_arrow
question_answer 21) In hydrogen atom, the electron is moving around the nucleus with the velocity \[2.18\times {{10}^{6}}m/s\]in an orbit of radios \[{{0.52}^{o}}\overset{\text{o}}{\mathop{\text{A}}}\,\]The acceleration of the electron is
A)
\[9\times {{10}^{18}}m/{{s}^{2}}\]
done
clear
B)
\[9\times {{10}^{22}}m/{{s}^{2}}\]
done
clear
C)
\[9\times {{10}^{-22}}m/{{s}^{2}}\]
done
clear
D)
\[9\times {{10}^{12}}m/{{s}^{2}}\]
done
clear
View Answer play_arrow
question_answer 22) When a spring is stretched by a distance \[x,\]it exerts a force given by \[F=(-5x-16{{x}^{3}})N\] The work done, when the spring is streached from 0.1m to 0.2m is
A)
\[8.7\times {{10}^{-2}}J\]
done
clear
B)
\[12.2\,\times {{10}^{-2}}J\]
done
clear
C)
\[8.1\times {{10}^{-1}}J\]
done
clear
D)
\[12.2\times {{10}^{-1}}J\]
done
clear
View Answer play_arrow
question_answer 23) A simple pendulum has a time period \[{{T}_{1}}\]when on the earths surface and \[{{T}_{2}}\]when taken to a height R above the earths surface, where R is the radius of earth. The value of \[\frac{{{T}_{2}}}{{{T}_{1}}}\]is
A)
1
done
clear
B)
\[\sqrt{2}\]
done
clear
C)
4
done
clear
D)
2
done
clear
View Answer play_arrow
question_answer 24) There is a clock which gives correct time at \[\text{20}{{\,}^{\text{o}}}\text{C}\]is subjected to \[\text{40}{{\,}^{\text{o}}}\text{C}\] The coefficient of linear expansion of the pendulum is \[12\times {{10}^{-6}}\]per \[{{\,}^{o}}C\] how much is gain or loss in time?
A)
10.3 s/day
done
clear
B)
19s/day
done
clear
C)
5.5 s/day
done
clear
D)
6.8 s/day
done
clear
View Answer play_arrow
question_answer 25) The excess pressure inside a soap bubble of radius 4 cm is \[30\,dyne/c{{m}^{2}}.\] The surface tension is
A)
30 dyne/cm
done
clear
B)
20 dyne/cm
done
clear
C)
40 dyne/cm
done
clear
D)
80 dyne/cm
done
clear
View Answer play_arrow
question_answer 26) If a body moves through a distance greater than \[2\pi R\]in one full rotation. Then,
A)
\[{{V}_{cm}}>R\omega \]
done
clear
B)
\[{{V}_{cm}}<R\omega \]
done
clear
C)
\[{{V}_{cm}}\ge R\omega \]
done
clear
D)
\[{{V}_{cm}}\le R\omega \]
done
clear
View Answer play_arrow
question_answer 27) If the weight of a body in vacuum is \[\omega .{{\omega }_{1}}\] and \[{{\omega }_{2}}\]are the weights when it is immersed in a liquid of specific gravity\[{{\rho }_{1}}\] and \[{{\rho }_{2}}\] E respectively, then find the relation among\[{{\omega }_{1}},{{\omega }_{2}}\]and \[{{\omega }_{2}}\].
A)
\[\frac{{{w}_{1}}{{\rho }_{1}}-{{w}_{2}}{{\rho }_{2}}}{{{w}_{1}}+{{w}_{2}}}\]
done
clear
B)
\[{{w}_{1}}=\frac{{{w}_{1}}{{\rho }_{2}}-{{w}_{2}}{{\rho }_{2}}}{{{\rho }_{2}}-{{\rho }_{1}}}\]
done
clear
C)
\[w=\frac{{{w}_{1}}{{\rho }_{1}}+{{w}_{2}}{{\rho }_{2}}}{{{\rho }_{1}}+{{\rho }_{2}}}\]
done
clear
D)
\[w=\frac{{{w}_{1}}{{\rho }_{1}}+{{w}_{2}}{{\rho }_{1}}}{{{\rho }_{1}}+{{\rho }_{2}}}\]
done
clear
View Answer play_arrow
question_answer 28) A body of mass \[m=10\,kg\]is attached to a wire of length 0.3 m. Calculate the maximum angular velocity with which it can be rotated in a horizontal circle (Breaking stress of wire\[=4.8\times {{10}^{7}}N/{{m}^{2}}\] and area of cross-section of a wire\[={{10}^{-6}}{{m}^{2}}\])
A)
4 rad/s
done
clear
B)
8 rad/s
done
clear
C)
1 rad/s
done
clear
D)
2 rad/s
done
clear
View Answer play_arrow
question_answer 29) If the energy (E), velocity \[(\upsilon )\] and force (F) be taken as fundamental quantity, then the dimension of mass will be
A)
\[F{{v}^{-2}}\]
done
clear
B)
\[F{{v}^{-1}}\]
done
clear
C)
\[E{{v}^{-2}}\]
done
clear
D)
\[E{{v}^{2}}\]
done
clear
View Answer play_arrow
question_answer 30) In an \[n-p-n\]transistor
A)
negative charge moves from collector to base
done
clear
B)
negative charge moves from emitter to base
done
clear
C)
holes moves from base to collector
done
clear
D)
holes move from emitter to base
done
clear
View Answer play_arrow
question_answer 31) A conducting sphere of radius \[R=20\,cm\]is given a charge \[Q=16\mu C.\] What is E at its centre?
A)
\[3.6\times {{10}^{6}}\,N/C\]
done
clear
B)
\[1.8\times {{10}^{6}}\,N/C\]
done
clear
C)
Zero
done
clear
D)
\[0.9\times {{10}^{6}}N/C\]
done
clear
View Answer play_arrow
question_answer 32)
A coil having N turns carry a current as shown in the figure. The magnetic field intensity at point P is
A)
\[\frac{{{\mu }_{0}}Ni{{R}^{2}}}{2{{({{R}^{2}}+{{x}^{2}})}^{3/2}}}\]
done
clear
B)
\[\frac{{{\mu }_{0}}Ni}{2R}\]
done
clear
C)
\[\frac{{{\mu }_{0}}Ni{{R}^{2}}}{{{(R+x)}^{2}}}\]
done
clear
D)
zero
done
clear
View Answer play_arrow
question_answer 33) A car starts from rest, moves with an acceleration a and then decelerates at a constant rate b for sometime to come to rest. If the total time taken is t. The maximum velocity of car is given by
A)
\[\frac{abt}{(a+b)}\]
done
clear
B)
\[\frac{{{a}^{2}}t}{a+b}\]
done
clear
C)
\[\frac{at}{(a+b)}\]
done
clear
D)
\[\frac{{{b}^{2}}t}{a+b}\]
done
clear
View Answer play_arrow
question_answer 34) An organ pipe closed at one end has fundamental frequency of 1500 Hz. The maximum number of overtones generated by this pipe which a normal person can he or is
A)
4
done
clear
B)
13
done
clear
C)
6
done
clear
D)
9
done
clear
View Answer play_arrow
question_answer 35) Two planets are revolving around the earth with velocities \[{{\upsilon }_{1}}\]and \[{{\upsilon }_{2}}\]and in radii\[{{r}_{1}}\] and \[{{r}_{2}}({{r}_{1}}>{{r}_{2}})\] respectively. Then
A)
\[{{v}_{1}}={{v}_{2}}\]
done
clear
B)
\[{{v}_{1}}>{{v}_{2}}\]
done
clear
C)
\[{{v}_{1}}<{{v}_{2}}\]
done
clear
D)
\[\frac{{{v}_{1}}}{{{r}_{1}}}=\frac{{{v}_{2}}}{{{r}_{2}}}\]
done
clear
View Answer play_arrow
question_answer 36)
Output W is given by
A)
\[(X+Y)Z\]
done
clear
B)
\[(\bar{X}+\bar{Y})Z\]
done
clear
C)
\[\bar{X}.\bar{Y}+\bar{Z}\]
done
clear
D)
\[(\bar{X}.\bar{Y}).Z\]
done
clear
View Answer play_arrow
question_answer 37) In double slit experiment, the distance between two slits is 0.6 mm and these are illuminate with light of wavelength \[4800\overset{\text{o}}{\mathop{\text{A}}}\,.\] The angular width of first dark fringe on the screen distance 120 cm from slits will be
A)
\[8\times {{10}^{-4}}\,rad\]
done
clear
B)
\[6\times {{10}^{-4}}\,rad\]
done
clear
C)
\[4\times {{10}^{-4}}\,rad\]
done
clear
D)
\[16\times {{10}^{-4}}\,rad\]
done
clear
View Answer play_arrow
question_answer 38) Green light causes emission of photoelectron from a surface, but not the yellow light. Emission of photoelectron will occur if the surface is illuminated by
A)
microwave
done
clear
B)
red rays
done
clear
C)
ultraviolet rays
done
clear
D)
infrared rays
done
clear
View Answer play_arrow
question_answer 39) The kinetic energy of 1 g molecule of a gas at normal temperature and pressure is\[(R=8.31\,J/mol-K)\]
A)
\[3.4\times {{10}^{3}}J\]
done
clear
B)
\[2.97\times {{10}^{3}}J\]
done
clear
C)
\[1.2\times {{10}^{2}}J\]
done
clear
D)
\[0.66\times {{10}^{4}}J\]
done
clear
View Answer play_arrow
question_answer 40) Plate current will be maximum when
A)
both the grid and anode are negative
done
clear
B)
both the grid and anode are positive
done
clear
C)
grids is positive and anode is negative
done
clear
D)
grid is negative and anode is positive
done
clear
View Answer play_arrow
question_answer 41) Among the following properties describing diamagnetism identify the property that is wrongly stated.
A)
Diamagnetic material do not have permanent magnetic moment
done
clear
B)
Diamagnetism is explained in terms of electromagnetic induction
done
clear
C)
Diamagnetic materials have a small positive susceptibility
done
clear
D)
The magnetic moment of individual electrons neutralize each other.
done
clear
View Answer play_arrow
question_answer 42) The induction coil works on the principle of
A)
self-induction
done
clear
B)
mutual induction
done
clear
C)
ampenrs rule
done
clear
D)
Flemings right hand rule
done
clear
View Answer play_arrow
question_answer 43) The square root of the product of inductance and capacitance has the dimension of
A)
length
done
clear
B)
mass
done
clear
C)
time
done
clear
D)
no dimension
done
clear
View Answer play_arrow
question_answer 44) The optical path difference is defined as \[\Delta x=\frac{2\pi }{\lambda }.\]what are dimensions of optical path difference?
A)
\[{{M}^{0}}{{L}^{-1}}{{T}^{0}}\]
done
clear
B)
\[[{{M}^{1}}{{L}^{-1}}{{T}^{0}}]\]
done
clear
C)
\[[M{{L}^{0}}{{T}^{2}}]\]
done
clear
D)
\[[M{{L}^{-2}}T]\]
done
clear
View Answer play_arrow
question_answer 45) A force\[F=2\hat{i}+3\hat{i}+\hat{k}\] are on a body. The work done by the force for a displacement of \[-2i+\hat{j}-k\] is
A)
2 unit
done
clear
B)
4 unit
done
clear
C)
-2 unit
done
clear
D)
-4 unit
done
clear
View Answer play_arrow
question_answer 46) At an instant t, the coordinate of a particles are \[x=a{{t}^{2}},y=b{{t}^{2}}\]and \[z=0.\]The magnitude of velocity of particle at an instant t is
A)
\[t\sqrt{{{a}^{2}}+{{b}^{2}}}\]
done
clear
B)
\[\frac{v}{\sqrt{2}}\]
done
clear
C)
\[\frac{v}{\sqrt{3}}\]
done
clear
D)
\[2t\sqrt{{{a}^{2}}+{{b}^{2}}}\]
done
clear
View Answer play_arrow
question_answer 47) A body of mass 10 kg is to be raised by a massless string tram rest to rest, through a height 9.8 m. The greatest tension which the string can safely bear is 20 kg wt. The least time of ascent is
A)
1s
done
clear
B)
3s
done
clear
C)
4 s
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 48) A ball moving with a certain velocity hits another identical ball at rest. If the plane is frictionless and collision is elastic, the angle between the directions in which the balls move after collision, will be
A)
\[{{30}^{o}}\]
done
clear
B)
\[{{60}^{o}}\]
done
clear
C)
\[{{90}^{o}}\]
done
clear
D)
\[{{120}^{o}}\]
done
clear
View Answer play_arrow
question_answer 49) A 70 kg man standing on ice through a B kg body horizontally at 8 m/s. The friction coefficient between the ice and his feet is 0.02. The distance, the man slips is
A)
0.3 m
done
clear
B)
2m
done
clear
C)
1 m
done
clear
D)
100
done
clear
View Answer play_arrow
question_answer 50) Pressure inside two soap bubble are 1.01 and 1.03 aim, ratio between their volume is
A)
27:1
done
clear
B)
3:1
done
clear
C)
127:101
done
clear
D)
None of these
done
clear
View Answer play_arrow
question_answer 51) The rate law for the reaction, \[2X+Y\to Z\]is \[Rate=k\,[X][Y].\]The correct statement with regard to this relation is
A)
the rate of reaction is independent of [X] and [V]
done
clear
B)
for this reaction \[{{t}_{1/2}}\] is independent of initial concentration of reactants
done
clear
C)
the unit of \[k\] is \[{{S}^{-1}}\]
done
clear
D)
the rate of formation of Z is half the rate of disappearance of X
done
clear
View Answer play_arrow
question_answer 52) If the activation energy for the forward reaction is \[250\,kJ\,mo{{l}^{-1}}\] and that of the reverse reaction is \[360\,kJ\,mo{{l}^{-1}},\] what is the enthalpy change for the reaction?
A)
\[+\,110\,k\,mo{{l}^{-1}}\]
done
clear
B)
\[-\,110\,kJ\,mo{{l}^{-1}}\]
done
clear
C)
\[+\,610\,kJ\,mo{{l}^{-1}}\]
done
clear
D)
\[-\,610\,kJ\,mo{{l}^{-1}}\]
done
clear
E)
None option is correct
done
clear
View Answer play_arrow
question_answer 53) Which one of the following statement is correct about lyophilic sols?
A)
These are formed by inorganic substances
done
clear
B)
These are irreversible
done
clear
C)
These are readily coagulated by addition of electrolytes
done
clear
D)
These are self-stabilized
done
clear
View Answer play_arrow
question_answer 54) Which one of the following is true in respect of adsorption?
A)
\[\Delta G<0,\Delta S<0,\Delta H<0\]
done
clear
B)
\[\Delta G<0,\Delta S>0,\Delta H<0\]
done
clear
C)
\[\Delta G>0,\Delta S>0,\Delta H>0\]
done
clear
D)
\[\Delta G<0,\Delta S<0,\Delta H>0\]
done
clear
View Answer play_arrow
question_answer 55) In extraction of chlorine by electrolysis of brine
A)
oxidation of CF ion to chlorine gas occurs
done
clear
B)
reduction of CF ion to chlorine gas occurs
done
clear
C)
for overall reaction AG° has negative value
done
clear
D)
a displacement reaction taken place
done
clear
View Answer play_arrow
question_answer 56) Which of the following does not exist in free form?
A)
\[B{{H}_{3}}\]
done
clear
B)
\[B{{F}_{3}}\]
done
clear
C)
\[BC{{l}_{3}}\]
done
clear
D)
\[BB{{r}_{3}}\]
done
clear
View Answer play_arrow
question_answer 57) Which of the following on thermal decomposition yields a basic as well as an acidic oxide?
A)
\[NaN{{O}_{3}}\]
done
clear
B)
\[BeC{{O}_{3}}\]
done
clear
C)
\[CaC{{O}_{3}}\]
done
clear
D)
\[KCl{{O}_{3}}\]
done
clear
View Answer play_arrow
question_answer 58) The shape of \[{{O}_{2}}{{F}_{2}}\]is similar to that of
A)
\[{{C}_{2}}{{F}_{2}}\]
done
clear
B)
\[{{H}_{2}}{{O}_{2}}\]
done
clear
C)
\[N{{a}_{2}}{{O}_{2}}\]
done
clear
D)
\[{{C}_{2}}{{H}_{2}}\]
done
clear
View Answer play_arrow
question_answer 59) Caros acid is also known as
A)
dithionic acid, \[{{H}_{2}}{{S}_{2}}{{O}_{6}}\]
done
clear
B)
sulphurous acid, \[{{H}_{2}}S{{O}_{3}}\]
done
clear
C)
peroxomono sulphuric acid,\[{{H}_{2}}S{{O}_{5}}\]
done
clear
D)
pyro sulphuric acid, \[{{H}_{2}}{{S}_{2}}{{O}_{7}}\]
done
clear
View Answer play_arrow
question_answer 60) A certain metal sulphide, \[M{{s}_{2}}\]is used extensively as a high temperature lubricant. If \[M{{s}_{2}}\]is 40.06% by mass sulphur, metal M has atomic mass
A)
160u
done
clear
B)
40 u
done
clear
C)
64 u
done
clear
D)
96 u
done
clear
View Answer play_arrow
question_answer 61) In which of the following molecules would you expect the nitrogen to nitrogen bond to be shortest?
A)
\[{{N}_{2}}{{H}_{4}}\]
done
clear
B)
\[{{N}_{2}}\]
done
clear
C)
\[{{N}_{2}}{{O}_{4}}\]
done
clear
D)
\[{{N}_{2}}O\]
done
clear
View Answer play_arrow
question_answer 62) Two of the following species have the same shape. Which are these two? \[N{{I}_{3}},I_{3}^{-},SO_{3}^{2-},NO_{3}^{-}\]
A)
\[N{{I}_{3}},I_{3}^{-}\]
done
clear
B)
\[I_{3}^{-},SO_{3}^{2-}\]
done
clear
C)
\[N{{I}_{3}},SO_{3}^{2-}\]
done
clear
D)
\[I_{3}^{-},NO_{3}^{-}\]
done
clear
View Answer play_arrow
question_answer 63) Select incorrect statement.
A)
Double bond is shorter than a single bond
done
clear
B)
x\[\sigma -\]bond is weaker than a \[\pi -\]bond
done
clear
C)
Double bond is stronger than a single bond
done
clear
D)
Covalent bond is stronger than a hydrogen bond
done
clear
View Answer play_arrow
question_answer 64) . Which is the correct order of size of \[{{O}^{-}},{{O}^{2-}},{{F}^{-}}\]and F?
A)
\[{{O}^{2-}}>{{O}^{-}}>{{F}^{-}}>F\]
done
clear
B)
\[{{O}^{-}}>{{O}^{2-}}>{{F}^{-}}>F\]
done
clear
C)
\[{{O}^{2-}}>{{F}^{-}}>F>{{O}^{-}}\]
done
clear
D)
\[{{O}^{2-}}>{{F}^{-}}>{{O}^{-}}>F\]
done
clear
View Answer play_arrow
question_answer 65) A sulphur containing species that cant be a reducing agent is
A)
\[S{{O}_{2}}\]
done
clear
B)
\[SO_{3}^{2-}\]
done
clear
C)
\[{{H}_{2}}S{{O}_{4}}\]
done
clear
D)
\[{{S}^{2-}}\]
done
clear
View Answer play_arrow
question_answer 66) A process that produces a one unit increase in atomic number is
A)
electron capture
done
clear
B)
\[\beta -\]emission
done
clear
C)
\[\alpha -\]emission
done
clear
D)
\[\gamma -\]ray emission
done
clear
View Answer play_arrow
question_answer 67) How many milliliters of \[0.45\,\text{MBaC}{{\text{l}}_{\text{2}}}\]solution contain 15.0 g of \[BaC{{l}_{2}}(208\,g\,mo{{l}^{-1}})\]
A)
110.7mL
done
clear
B)
124.6mL
done
clear
C)
135.8 mL
done
clear
D)
160.3 Ml
done
clear
View Answer play_arrow
question_answer 68) Choose correct statement.
A)
\[{{H}_{2}}\]is more rapidly adsorbed on to the surf then \[{{D}_{2}}\]
done
clear
B)
\[{{H}_{2}}\]reacts over 13 times faster with \[C{{l}_{2}}\]than because \[{{D}_{2}}\]has a lower energy of activation
done
clear
C)
Both [a] and [b] are true
done
clear
D)
None of the above is true
done
clear
View Answer play_arrow
question_answer 69) Amongst the following, identify the species with an atom in + 6 oxidation state.
A)
\[MnO_{4}^{-}\]
done
clear
B)
\[Cr(CN)_{6}^{3-}\]
done
clear
C)
\[NiF_{6}^{2-}\]
done
clear
D)
\[Cr{{O}_{2}}C{{l}_{2}}\] In \[Cr{{O}_{2}}C{{l}_{2}},Cr\]is in + 6 oxidation state.
done
clear
View Answer play_arrow
question_answer 70) The radii of the elements from chromium (Z =24) to copper (Z = 29) are very close to one another. This is due to
A)
lanthanide contraction
done
clear
B)
the fact that successive addition of d -electrons screen the outer electrons (4s-) from the inward pull of the nucleus
done
clear
C)
increase in radii due to increase in n is compensated by decrease in radii due to increase in Z
done
clear
D)
atomic radii do not remain constant but decrease in a normal gradation
done
clear
View Answer play_arrow
question_answer 71) The octahedral complex ion \[{{[FeC{{l}_{6}}]}^{3-}}\]
A)
5 unpaired electrons \[(t_{2g}^{3}e_{g}^{2})\]
done
clear
B)
1 unpaired electron \[(t_{2g}^{1})\]
done
clear
C)
3 unpaired electrons \[(t_{2g}^{1}e_{g}^{2})\]
done
clear
D)
All the electrons are paired
done
clear
View Answer play_arrow
question_answer 72) The complex \[{{[Co{{(N{{H}_{3}})}_{5}}(N{{O}_{2}})]}^{2+}}\] and \[{{[Co{{(N{{H}_{3}})}_{5}}(ONO)]}^{2+}}\] are called
A)
ionisation isomers
done
clear
B)
linkage isomers
done
clear
C)
coordination isomers
done
clear
D)
geometrical isomers
done
clear
View Answer play_arrow
question_answer 73) If \[Z{{n}^{2+}}|Zn\]electrode is diluted 100 times, then the change in emf is
A)
increase of 59 mV
done
clear
B)
decrease of 59 mV
done
clear
C)
increase of 29.5 mV
done
clear
D)
decrease of 29.5 mV
done
clear
View Answer play_arrow
question_answer 74) At 291 K the molar conductivities at infinite dilution of \[N{{H}_{4}}Cl,NaOH\]and \[NaCI\]are 129.8, 217.4 and \[108.9\,Sc{{m}^{2}}\]respectively,. If the molar conductivity of a centinormal solution of \[N{{H}_{4}}OH\]is \[9.33\,Sc{{m}^{2}},\] What is the precentage dissociation of \[N{{H}_{4}}OH\]at this dilution?
A)
1.76%
done
clear
B)
2.28%
done
clear
C)
3.92%
done
clear
D)
4.15%
done
clear
View Answer play_arrow
question_answer 75) On chemical analysis, it is found that 200 mL of\[CaC{{l}_{2}}\] solution contains \[3.01\times {{10}^{22}}\] chloride ions. Calculate the molarity of this solution.
A)
O.125M
done
clear
B)
0.178M
done
clear
C)
0.21 OM
done
clear
D)
0.253 M
done
clear
View Answer play_arrow
question_answer 76) The osmotic pressure of a urea solution is 500 mm of Hg at \[10{{\,}^{o}}C.\] The solution is diluted and its temperature is raised to \[25{{\,}^{o}}C\] It is now found that osmotic pressure of the solution is reduced to 105.3 mm of Hg. The extent of dilution of the solution is
A)
2 times
done
clear
B)
3 times
done
clear
C)
4 times
done
clear
D)
5 times
done
clear
View Answer play_arrow
question_answer 77) Iron changes its crystal structure from body centred to cubic close packed structure when heated to 91\[916{{\,}^{o}}C\]. Calculate the ratio of the density of the bcc crystal to that of ccp crystal, assuming that the metallic radius of the atom does not change
A)
0.639
done
clear
B)
0.743
done
clear
C)
0.896
done
clear
D)
0.919
done
clear
View Answer play_arrow
question_answer 78) An ionic compound made up of atoms A and B has fee arrangement in which atoms A are at the corners and atoms B are at the face-centres. If one of the atoms is missing from the corner, the simplest formula of the compound will be
A)
\[{{A}_{7}}{{B}_{24}}\]
done
clear
B)
\[{{A}_{2}}{{B}_{3}}\]
done
clear
C)
\[{{A}_{3}}{{B}_{7}}\]
done
clear
D)
\[{{A}_{3}}{{B}_{10}}\]
done
clear
View Answer play_arrow
question_answer 79) Which of the following is halogen exchange reaction?
A)
\[RX+Nal\xrightarrow{{}}Rl+NaX\]
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 80) Chlorobenzene is formed by the reaction of chlorine with benzene in the presence of \[AlC{{l}_{3}}.\]Which of the following species attacks the benzene ring in this reaction?
A)
\[C{{l}^{-}}\]
done
clear
B)
\[C{{l}^{+}}\]
done
clear
C)
\[AlC{{l}_{3}}\]
done
clear
D)
\[{{[AlC{{l}_{4}}]}^{-}}\]
done
clear
View Answer play_arrow
question_answer 81) The conversion of m-mitrophenol to resorcinol involves respectively
A)
reduction, diazotisation, hydrolysis
done
clear
B)
hydrolysis, reduction, diazotisation
done
clear
C)
hydrolysis, diazotisation, reduction
done
clear
D)
diazotisation, reduction, hydrolysis
done
clear
View Answer play_arrow
question_answer 82) Formation of methyl tertiary butyl ether by the reaction of sodium tertiary butoxide and methyl bromide involves
A)
elimination reaction
done
clear
B)
electrophilic addition reaction
done
clear
C)
nucleophilic addition reaction
done
clear
D)
nucleophilic substitution reaction
done
clear
View Answer play_arrow
question_answer 83) In Clemmensen reduction, carbonyl compound is treated with
A)
Zn amalgam \[\text{+ HCI}\]
done
clear
B)
Na amalgam\[\text{ }\!\!~\!\!\text{ + HCI}\]
done
clear
C)
Zn amalgam \[\text{+}\,\text{HN}{{\text{O}}_{\text{3}}}\]
done
clear
D)
Na amalgam \[\text{HN}{{\text{O}}_{\text{3}}}\]
done
clear
View Answer play_arrow
question_answer 84) An organic compound [A] with molecular formula \[{{\text{C}}_{8}}{{H}_{8}}O\]forms an orange red precipitate with 2, 4-DNP reagent and gives yellow precipitate on heating with iodine in the presence of sodium hydroxide. It neither reduces Tollens reagent or Fehlings reagent, nor decolourises bromine water or Baeyers reagent. Compound A is
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 85) Which of the following sets consist only of essential amino acids?
A)
Alanine, lysine, glutamine
done
clear
B)
Lysine, leucine, tryptophane
done
clear
C)
Tyrosine, cysteine,alanine
done
clear
D)
Proline, glycine, leucine
done
clear
View Answer play_arrow
question_answer 86) Which of the following reactions of glucose can be explained only by its cyclic structure?
A)
Glucose forms pentaacetate
done
clear
B)
Glucose is oxidised by nitric acid to gluconic acid
done
clear
C)
Glucose reacts with hydroxyl amine to form an oxime
done
clear
D)
Pentaacetate of glucose does not react with hydroxyl amine
done
clear
View Answer play_arrow
question_answer 87) Which one of the following tranquilisers is not a derivative of barbituric acid?
A)
Veronal
done
clear
B)
Seconal
done
clear
C)
Luminal
done
clear
D)
Equanil
done
clear
View Answer play_arrow
question_answer 88) Arrange the following alkenes towards order of increasing reactivity in cationic polymerisation \[{{H}_{2}}C=CHC{{H}_{3}},{{H}_{2}}C=CHCl,\]\[{{H}_{2}}C=\underset{III}{\overset{I}{\mathop{C}}}\,H{{C}_{6}}{{H}_{5}},{{H}_{2}}C=\underset{(IV)}{\mathop{CHCOOC{{H}_{3}}}}\,\]
A)
IV < II < I < III
done
clear
B)
111 < II < I < IV
done
clear
C)
I < II < III < IV
done
clear
D)
II < IV < I < III
done
clear
View Answer play_arrow
question_answer 89) Which of the following electronic transitions requires that the greatest quantity of energy be absorbed by a hydrogen atom?
A)
n = 1 to n = 2
done
clear
B)
n = 2 to n = 4
done
clear
C)
n= 3ton= 6
done
clear
D)
n = 1 to n = \[\infty \]
done
clear
View Answer play_arrow
question_answer 90) If angular momentum quantum number can take value of n also (in addition to other possible values) then total number of electrons in first orbit would have been
A)
2
done
clear
B)
6
done
clear
C)
8
done
clear
D)
10
done
clear
View Answer play_arrow
question_answer 91) A quantity of hydrogen gas occupies a volume of 30.0 mL at a certain temperature and pressure. What volume would half this mass of hydrogen occupy at triple the absolute temperature if the pressure were one-ninth that of the original gas?
A)
270 mL
done
clear
B)
90 mL
done
clear
C)
405 mL
done
clear
D)
135 mL
done
clear
View Answer play_arrow
question_answer 92) For the following equilibrium, \[{{N}_{2}}{{O}_{4}}2N{{O}_{2}};{{K}_{c}}=0.67.\]If we start with 3 moles of \[N{{O}_{2}}\]and 1 mole of \[{{N}_{2}}{{O}_{4}}\]in 1L flask, then \[N{{O}_{2}}\]present at equilibrium is
A)
1.5 mol
done
clear
B)
2.0 mol
done
clear
C)
0.5 mol
done
clear
D)
1.0 mol
done
clear
View Answer play_arrow
question_answer 93) \[(\Delta H-\Delta E)\] is maximum at a given temperaute in case of
A)
\[PC{{l}_{5}}(g)\xrightarrow{{}}PC{{l}_{3}}(g)+C{{l}_{2}}(g)\]
done
clear
B)
\[CaC{{O}_{3}}(s)\xrightarrow{{}}CaO(s)+C{{O}_{2}}(g)\]
done
clear
C)
\[N{{H}_{4}}HS(s)\xrightarrow{{}}N{{H}_{3}}(g)+{{H}_{2}}S(g)\]
done
clear
D)
\[{{N}_{2}}(g)+{{O}_{2}}(g)\xrightarrow{{}}2NO(g)\]
done
clear
View Answer play_arrow
question_answer 94) Which of the following is spontaneous?
A)
if fusion of perfume molecules from one side of the room to other
done
clear
B)
Decomposition of solid \[CaC{{O}_{3}}\]
done
clear
C)
Heat flow from a cold object to a hot object
done
clear
D)
Climbing up a mountain.
done
clear
View Answer play_arrow
question_answer 95) On burning 4.0 g of iron to ferric oxide at constant pressure, the heat evolved is 29.28 kJ. The enthalpy of formation of ferric oxide is (At\[\omega t.Fe=56\])
A)
\[81.98\text{ }kJ\text{ }mo{{1}^{-1}}\]
done
clear
B)
\[-\text{ }81.98\text{ }kJ\text{ }mo{{1}^{-1}}\]
done
clear
C)
\[\text{ }819.8\text{ }kJ\text{ }mo{{1}^{-1}}\]
done
clear
D)
\[-819.8\,kJ\,mo{{l}^{-1}}\]
done
clear
View Answer play_arrow
question_answer 96) A saturated solution prepared by dissolving \[A{{g}_{2}}C{{O}_{3}}\]in water has \[[A{{g}^{+}}]=2.56\times {{10}^{-4}}M.\]Hence, its \[{{K}_{sp}}\]is
A)
\[8.4\times {{10}^{-12}}\]
done
clear
B)
\[1.68\times {{10}^{-13}}\]
done
clear
C)
\[6.6\times {{10}^{-8}}\]
done
clear
D)
\[1.6\times {{10}^{-2}}\]
done
clear
View Answer play_arrow
question_answer 97) In group III analysis, buffer used to precipitate cations as hydroxide is
A)
\[N{{H}_{4}}Cl+N{{H}_{4}}OH\]
done
clear
B)
\[HCO_{3}^{-}+C{{O}_{2}}\]
done
clear
C)
\[C{{H}_{3}}COOH+C{{H}_{3}}COONa\]
done
clear
D)
\[{{C}_{6}}{{H}_{5}}COOH+{{C}_{6}}{{H}_{5}}COONa\]
done
clear
View Answer play_arrow
question_answer 98) Which one of the following product is most expected?
A)
done
clear
B)
done
clear
C)
done
clear
D)
done
clear
View Answer play_arrow
question_answer 99) On the basis of the fact that \[NaN{{H}_{2}}\]can deprotonate \[HC\equiv CH\]but not \[C{{H}_{2}}=C{{H}_{2}}\]and the hybridization effect, order the following. four acids according to acidity \[\underset{I}{\mathop{HC\equiv CH}}\,\underset{II}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\underset{III}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,\underset{IV}{\mathop{N{{H}_{3}}}}\,\]
A)
IV < III < II < I
done
clear
B)
III < 11 < IV < I
done
clear
C)
I < IV < II < III
done
clear
D)
III < IV < II < I
done
clear
View Answer play_arrow
question_answer 100) Amides are less basic than amines because
A)
a carbonyl group, donates electrons by resonance
done
clear
B)
the carbonyl group withdraws electrons by resonance
done
clear
C)
nitrogen does not have a lone pair of electrons
done
clear
D)
nitrogen has a full positive charge
done
clear
View Answer play_arrow
question_answer 101) Mendels pattern of inheritance systematically showed the progeny in
A)
square board
done
clear
B)
cross board
done
clear
C)
checker board
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 102) When a negro marries white, how many phenotypes are obtained?
A)
7
done
clear
B)
8
done
clear
C)
10
done
clear
D)
16
done
clear
View Answer play_arrow
question_answer 103) It is easier to engineer plants genetically as compared to animals because
A)
E. coli plasmids can serve as vectors in plant
done
clear
B)
Plants can be grown from a single cell in tissue culture
done
clear
C)
Agriculturally significant plants have been engineered
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 104) Assertion [A] Burgundy mixture is also called soda bordeaux. Reason [R] Burgundy mixture is a modification of bordeaux mixture containing sodium phosphate instead of lime.
A)
Both A and R are correct and the R is a correct explanation of A
done
clear
B)
Both A and R are correct, but R is not a correct explanation of A
done
clear
C)
A is true, but R is false
done
clear
D)
Both A and R are false
done
clear
View Answer play_arrow
question_answer 105) Carina is
A)
outermost two petals of pea flower
done
clear
B)
a boat shaped structure of petals
done
clear
C)
the innermost two petals that are appressed together
done
clear
D)
Both [b] and [c]
done
clear
View Answer play_arrow
question_answer 106) What should be the genotype of a round seeded tall plant, which when crossed with a plant of similar genotype produces the following percentage of phenotypes? (tall round = 56.26% tall wrinkled = 18.75%, dwarf round = 18.75% dwarf wrinkled =6.25%)
A)
TtRr
done
clear
B)
TTrr
done
clear
C)
TTRr
done
clear
D)
TtRR
done
clear
View Answer play_arrow
question_answer 107) Gossypium hirsutum is
A)
old world tetraploid
done
clear
B)
new world tetraploid
done
clear
C)
old world diploid
done
clear
D)
new world diploid
done
clear
View Answer play_arrow
question_answer 108) Heart of cockroach is
A)
6-chambered
done
clear
B)
\[9-\] chambered
done
clear
C)
29-chambered
done
clear
D)
13-chambered
done
clear
View Answer play_arrow
question_answer 109)
Math the following simple epithelial tissues in column I with their occurrence in column II and choose the correct combination from the options give. Column I Column II A. Squamous 1. Intestinal glands B. Cuboidal 2. Trachea C. Columnar 3. Ovary D. Ciliated 4. Alveoli E. Pseudostratified 5. Bronchioles
A)
A-5 B-4 C-1 D-2 E-3
done
clear
B)
A-1 B-2 C-4 D-3 E-5
done
clear
C)
A-4 B-3 C-1 D-5 E-2
done
clear
D)
A-4 B-5 C-1 D-2 E-3
done
clear
View Answer play_arrow
question_answer 110) The most appropriate definition of neuroglial cell are that they are
A)
secretory cell
done
clear
B)
compound alveolar gland
done
clear
C)
non-sensory and supporting cell
done
clear
D)
sensory and supportinq cells
done
clear
View Answer play_arrow
question_answer 111) Potato is included in Solanaceae family because
A)
it is pentamerous
done
clear
B)
it is epipetalous
done
clear
C)
ovary is slightly diverted from its position
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 112) The nature of cell occurring in medulla is
A)
suberised
done
clear
B)
liquefied
done
clear
C)
collenchymatous
done
clear
D)
parenchymatous
done
clear
View Answer play_arrow
question_answer 113) In cockroach, the longest podomere is
A)
coxa
done
clear
B)
tarsus
done
clear
C)
tibia
done
clear
D)
femur
done
clear
View Answer play_arrow
question_answer 114) The embryonated egg of Ascaris represents
A)
an egg with a juvenile
done
clear
B)
an egg with blastula
done
clear
C)
an egg with gastrula
done
clear
D)
an egg with an egg
done
clear
View Answer play_arrow
question_answer 115) The smallest plant division-Gymnosperm has how many species.
A)
640
done
clear
B)
90C
done
clear
C)
100
done
clear
D)
300
done
clear
View Answer play_arrow
question_answer 116) Sequence of taxonomic categories is
A)
division, class, family, tribe, order, genus, species
done
clear
B)
class, phylum, tribe, order, family, genus, specie
done
clear
C)
division, class, order, family, tribe, genus, species
done
clear
D)
phylum, order, class, tribe, family, genus, species
done
clear
View Answer play_arrow
question_answer 117) Classification given by Bentham and Hooker is
A)
nature
done
clear
B)
artificial
done
clear
C)
numerical
done
clear
D)
phylogenetic
done
clear
View Answer play_arrow
question_answer 118) Which of the following produces spores, but lacks vasculature?
A)
Fungi
done
clear
B)
Dicots
done
clear
C)
Pteridophytes
done
clear
D)
Gymnosperms
done
clear
View Answer play_arrow
question_answer 119) The phylogenetic system of classification was put forth by
A)
Aristotle
done
clear
B)
Carolus Linnaeus
done
clear
C)
Theophrastus
done
clear
D)
Adolf Engler and Karl PrantI
done
clear
View Answer play_arrow
question_answer 120) Meiosis II performs
A)
synthesis of DNA and centromere
done
clear
B)
separation of sex-chromosomes
done
clear
C)
separatin of homologous chromosomes
done
clear
D)
speparation of chromatids
done
clear
View Answer play_arrow
question_answer 121)
Consider the following statements and select the correct option. I. ER helps in the transport of substances, synthesis of lipoproteins, proteins and glycogen. II. Ribosomes are involved in protein synthesis. III. Mitochondria helps in oxidative phosphorylation and generation of ATP IV. The endomembrane system includes ER, Golgi complex, lysosomes, plasma membrane and vacuoles.
A)
I, II and III are correct
done
clear
B)
I alone is correct
done
clear
C)
III alone is correct
done
clear
D)
II alone is correct
done
clear
View Answer play_arrow
question_answer 122) Find out the wrongly matched pair.
A)
Primary metabolite-Ribose
done
clear
B)
Secondary metabolite-Anthocyanins
done
clear
C)
Protein-Insulin
done
clear
D)
Cellulose-Heteropolymer
done
clear
View Answer play_arrow
question_answer 123) Which are of the following sets of characters are applicable in metamorphosis of tadpole larva of frog and toads?
A)
Resorption of gills and lengthening of tail
done
clear
B)
Complex development of gill and lengthening of tail
done
clear
C)
Resorption of gills and resorption of tail
done
clear
D)
Complex development of gill and resorption of tail
done
clear
View Answer play_arrow
question_answer 124)
Match column I and column II and choose the right option. Column I Column II A. Rhizopus 1. Ascomycetes B. Penicillium 2. Basidiomycetes C. Ustilago 3. Deuteromycetes D. Alternaha 4. Phycomycetes
A)
A-4 B-3 C-1 D-2
done
clear
B)
A-4 B-3 C-1 D-2
done
clear
C)
A-4 B-1 C-2 D-3
done
clear
D)
A-2 B-3 C-4 D-1
done
clear
View Answer play_arrow
question_answer 125) The name of Smt. Thimmakka is associated with the
A)
agitations against hydroelectric project
done
clear
B)
planting and conservation of avenue trees
done
clear
C)
Appiko movement
done
clear
D)
Conservation of fauna and flora of the Western ghats
done
clear
View Answer play_arrow
question_answer 126)
Match the items of column I with column II and select the correct option. Column I Column II A. Electrostatic Precipitator 1. Removes gases like\[S{{O}_{2}}\] B. Scrubber 2. Reduces Automobile emission C. Catalytic converter 3.Removes particulate matter
A)
A-3 B-1 C-2
done
clear
B)
A-2 B-3 C-1
done
clear
C)
A-1 B-2 C-3
done
clear
D)
A-3 B-2 C-1
done
clear
View Answer play_arrow
question_answer 127) In ecological succession the climax community is beat recognised by the following state.
A)
\[P>R\]
done
clear
B)
\[P\ne R\]
done
clear
C)
\[P=R\]
done
clear
D)
\[P<R\]
done
clear
View Answer play_arrow
question_answer 128) Sound above what level is considered hazardous noise pollution?
A)
Above 120 dB
done
clear
B)
Above 80 dB
done
clear
C)
Below 30 dB
done
clear
D)
Above 150 dB
done
clear
View Answer play_arrow
question_answer 129) Read the two statements A and B. Statement A Diversity observed in the entire geographical area, is called gamma diversity. Statement B Biodiversity decreases from high altitude to low altitude.
A)
Statements B is correct, A is wrong
done
clear
B)
Statements A is correct, B is wrong
done
clear
C)
Both the statements A and B are wrong
done
clear
D)
Both the statements A and B are correct
done
clear
View Answer play_arrow
question_answer 130) Green muffler is used against, which type of pollution?
A)
Soil
done
clear
B)
Air
done
clear
C)
Noise
done
clear
D)
Water
done
clear
View Answer play_arrow
question_answer 131) Black lung disease is common in
A)
coal miners
done
clear
B)
farmers
done
clear
C)
refinery workers
done
clear
D)
petrochemical industry
done
clear
View Answer play_arrow
question_answer 132) The purpose of biological treatment of waste water is to
A)
reduce BOD
done
clear
B)
increase BOD
done
clear
C)
increase sedimentation
done
clear
D)
reduce sedimentation
done
clear
View Answer play_arrow
question_answer 133) Pedology refers to
A)
study of water
done
clear
B)
study of fossils
done
clear
C)
study of soil
done
clear
D)
study of population
done
clear
View Answer play_arrow
question_answer 134) The aquatic organisms that can actively swim against the water current is
A)
plankton
done
clear
B)
benthos
done
clear
C)
neuston
done
clear
D)
nekton
done
clear
View Answer play_arrow
question_answer 135) Lichens are described as indicator of
A)
water pollution
done
clear
B)
air pollution
done
clear
C)
soil pollution
done
clear
D)
agriculture productivity
done
clear
View Answer play_arrow
question_answer 136) Ethyl alcohol is obtained commercially on a large scale from
A)
maize
done
clear
B)
bajra
done
clear
C)
grapes
done
clear
D)
sugar cane
done
clear
View Answer play_arrow
question_answer 137) Insect resistance transgenic cotton has been produced by inserting a piece of DNA from
A)
an insect
done
clear
B)
a virus
done
clear
C)
a wild relative of cotton
done
clear
D)
a bacterium
done
clear
View Answer play_arrow
question_answer 138) The technique of production of monoclonal antibodies was developed by
A)
Milstein and Kohler
done
clear
B)
Watson and Crick
done
clear
C)
Fredrick and Miescher
done
clear
D)
Bentham and Miescher
done
clear
View Answer play_arrow
question_answer 139) Find the incorrect statement.
A)
Calcitonin is a medically useful recombinant product in the treatment of infertility
done
clear
B)
Bt toxin is a biodegradable insecticide obtained from Bacillus thuringiensis
done
clear
C)
Trichoderma sp. Is a biocontrol agent for fungal diseases of plant
done
clear
D)
Gene therapy is a genetic engineering technique used to treat disease at molecular level by replacing defective genes with normal genes.
done
clear
View Answer play_arrow
question_answer 140) Which of the following DNA sequences qualifies be designated as a palindrome?
A)
\[5GACGAG-3\,3-CTGGTC-5\]
done
clear
B)
\[5-AGCGCT-3\,3-TCGCGA-5\]
done
clear
C)
\[3-GACCAG-5\]in one strand
done
clear
D)
\[5-GACCAG-3\]in one strand
done
clear
View Answer play_arrow
question_answer 141) GAATTC is the recognisation site for which of the following restriction endonuclease?
A)
Bam I
done
clear
B)
Hal III
done
clear
C)
Eco Rl
done
clear
D)
Hind III
done
clear
View Answer play_arrow
question_answer 142) Which of the following is produced by I genetically engineered bacteria?
A)
ADH
done
clear
B)
Insulin
done
clear
C)
Thyroxine
done
clear
D)
Glucagon
done
clear
View Answer play_arrow
question_answer 143) Aspergillus niger produces
A)
fumaric acid
done
clear
B)
acetic acid
done
clear
C)
lactic acid
done
clear
D)
citric acid and gluconic acid
done
clear
View Answer play_arrow
question_answer 144) cDNA is
A)
circular DNA
done
clear
B)
cloned DNA
done
clear
C)
recombinant DNA
done
clear
D)
formed by reverse transcriptase
done
clear
View Answer play_arrow
question_answer 145) Chagas disease is caused by
A)
T. rhodesiense
done
clear
B)
Trypanosoma cruzi
done
clear
C)
Leishmania donovanf
done
clear
D)
T. gambiense
done
clear
View Answer play_arrow
question_answer 146) Which of the following is correct?
A)
Diabets-Sugar free
done
clear
B)
Heart attack-Radiation therapy
done
clear
C)
Leukemia-Skin cancer
done
clear
D)
Rheumatic fever-Defective pacemaker
done
clear
View Answer play_arrow
question_answer 147) Which is a viral disease?
A)
Tetanus
done
clear
B)
Cholera
done
clear
C)
Measles
done
clear
D)
Tuberculosis
done
clear
View Answer play_arrow
question_answer 148) The treatment of cancer with X-rays or gamma rays is called
A)
surgery
done
clear
B)
chemotherapy
done
clear
C)
hormone therapy
done
clear
D)
radiation theraphy
done
clear
View Answer play_arrow
question_answer 149)
Match the column I (bacteria) with the column II (diseases) and select the correct option. Column I Column II A Treponema pallidum 1. Remove plague B. Yersinia pestis 2. Anthrok C. Bacillus anthracis 3. Syphilis D. Vibrio cholerae 4 Cholera
A)
A-4 B-3 C-1 D-2
done
clear
B)
A-3 B-1 C-2 D-4
done
clear
C)
A-1 B-3 C-2 D-4
done
clear
D)
A-2 B-3 C-1 D-4
done
clear
View Answer play_arrow
question_answer 150) Honey is
A)
neutral
done
clear
B)
alkaline
done
clear
C)
acidic
done
clear
D)
basic after some days
done
clear
View Answer play_arrow
question_answer 151) Wilsons disease occurs due to defective metabolism of
A)
\[M{{g}^{2+}}\]
done
clear
B)
\[Z{{n}^{2+}}\]
done
clear
C)
\[F{{e}^{2+}}\]
done
clear
D)
\[C{{u}^{2+}}\]
done
clear
View Answer play_arrow
question_answer 152) Emasculation means
A)
removal of anthers
done
clear
B)
removal of pistils
done
clear
C)
removal of carpels
done
clear
D)
All of these
done
clear
View Answer play_arrow
question_answer 153)
Match column I with column II and choose the correct answer. Column I Column II A. Neoplasm 1. Haematopoietic cell tumours B. Benign tumour 2. Bone cartage, tissue cancer C. Carcinomas 3. Malignant tumour D. Sarcomas 4. Cancer of epithelid tissues E. Lymphomas 5. Non-cancerous tumour 6. Initiation bf new tumours
A)
A-6 B-4 C-3 D-2 E-1
done
clear
B)
A-6 B-3 C-4 D-2 E-1
done
clear
C)
A-3 B-5 C-4 D-2 E-1
done
clear
D)
A-3 B-5 C-4 D-2 E-3
done
clear
View Answer play_arrow
question_answer 154) Explants are
A)
uprooted plant parts for transpiration
done
clear
B)
plants collected after harvest
done
clear
C)
exploited parts of a plant
done
clear
D)
small parts grown for tissue culture
done
clear
View Answer play_arrow
question_answer 155) Kadiri-1, kadiri-2 are the varieties of
A)
red gram hybrids
done
clear
B)
rice hybrids
done
clear
C)
ground nut hybrids
done
clear
D)
sorghum hybrids
done
clear
View Answer play_arrow
question_answer 156) Which of the following is not matched correctly?
A)
Winged bean-Wood used for timber purposes
done
clear
B)
Guayle-Yields latex, which can be converted to rubber.
done
clear
C)
Jojoba-Seeds yields liquid wax
done
clear
D)
Leucaena-Wood is a source of timber, rayon and paper pulp.
done
clear
View Answer play_arrow
question_answer 157) Leucaena leucocephala is
A)
a small leguminous tree with edible fruits and seeds
done
clear
B)
a fodder plant as its pods and leaves are consumed by cattle
done
clear
C)
called subabul in India
done
clear
D)
All of the above
done
clear
View Answer play_arrow
question_answer 158) Pusa sawani is a hybrid variety of
A)
flat bean
done
clear
B)
maize
done
clear
C)
bhindi
done
clear
D)
Brassica
done
clear
View Answer play_arrow
question_answer 159) The pesticides are considered as hazardous because
A)
they affect the target organism only
done
clear
B)
they persist in the environment
done
clear
C)
they do not kill natural enemy population
done
clear
D)
they do not enter the food chain
done
clear
View Answer play_arrow
question_answer 160) What is Agent Orange?
A)
A biodegradable insecticide
done
clear
B)
A weedicide containing dioxin
done
clear
C)
Colour used in fluorescent lamps
done
clear
D)
Hazardous chemical used in luminous paints
done
clear
View Answer play_arrow
question_answer 161) The book Philosophic Zoologique is written by
A)
Linneaus
done
clear
B)
Malthus
done
clear
C)
Darwin
done
clear
D)
Lamarck
done
clear
View Answer play_arrow
question_answer 162) Improvement of human race is called
A)
human demography
done
clear
B)
human heridity
done
clear
C)
eugenics
done
clear
D)
euthenics
done
clear
View Answer play_arrow
question_answer 163) An environmental agent, which triggers transcription from an operon, is a
A)
inducer
done
clear
B)
regulator
done
clear
C)
depressor
done
clear
D)
controlling element
done
clear
View Answer play_arrow
question_answer 164) Acrosome is made up of
A)
centrioles
done
clear
B)
ribosomes
done
clear
C)
mitochondria
done
clear
D)
Golgi bodies
done
clear
View Answer play_arrow
question_answer 165) The mullerian duct in the female amniotes develops into
A)
oviduct
done
clear
B)
ureter
done
clear
C)
uterus .
done
clear
D)
seminal receptacle
done
clear
View Answer play_arrow
question_answer 166)
Which of the following statements is/are true? I. Endothecium lies behind epidermis. II. Fusion of egg with male gamete is called apogamy. III. Synergids are haploid. IV. The point at which funicle touches the ovule is raphe.
A)
I and II
done
clear
B)
I and III
done
clear
C)
Only I
done
clear
D)
I and IV
done
clear
View Answer play_arrow
question_answer 167)
Consider the following statements and choose the correct option. I. Rhizome in ginger serves as an organ of vegetative reproduction. II. The genetic constitution of a plant is unaffected in vegetative propagation. III. Totipotency of cells enables us to micropropagate plants.
A)
II and III are correct
done
clear
B)
I and III are correct
done
clear
C)
I, II and III are correct
done
clear
D)
Only I is correct
done
clear
View Answer play_arrow
question_answer 168)
Select the correct order of endosperm types.
A)
Free nuclear, cellular, helobial
done
clear
B)
Cellular, free nuclear, helobial
done
clear
C)
Cellular, helobial, free nuclear
done
clear
D)
Helobial, free nuclear, cellar
done
clear
View Answer play_arrow
question_answer 169) Double fertilisation in angiosperm plant means
A)
Fusion of egg cell twice with male gametes
done
clear
B)
Fusion of two egg cells with two male gametes
done
clear
C)
Fusion of one male gamete with the egg cell and the other male gamete with secondary nucleus
done
clear
D)
Fusion of one mate gamete with the egg cell and the other male gamete with the synergid
done
clear
View Answer play_arrow
question_answer 170) Xenogamy is
A)
pollination between two different flowers of same plant on same branch
done
clear
B)
a mechanism of parthenocarpy
done
clear
C)
pollination between anthers and stigma of same flowers
done
clear
D)
pollination between two flowers of tw0 different
done
clear
View Answer play_arrow
question_answer 171) Perisperm is
A)
persistent nucellus
done
clear
B)
peripheral part of endosperm
done
clear
C)
remnant of endosperm
done
clear
D)
disintegrated secondary nucleus
done
clear
View Answer play_arrow
question_answer 172) Malacophily is the name given to pollination by
A)
bats
done
clear
B)
snails
done
clear
C)
birds
done
clear
D)
insects
done
clear
View Answer play_arrow
question_answer 173) Generative cell was destroyed by laser but a normal pollen tube was still formed because
A)
laser beam stimulates growth of pollen tube
done
clear
B)
the region of emergence of pollen tube is not harmed
done
clear
C)
vegetative cell is not damaged
done
clear
D)
contents of killed generative cell stimulate pollen
done
clear
View Answer play_arrow
question_answer 174)
Refer the given figure and select the correct option.
A)
It is a type of asexual reproduction
done
clear
B)
It is a type of parthenogenesis
done
clear
C)
Both [a] and [b]
done
clear
D)
The off springs can also be called as clone
done
clear
View Answer play_arrow
question_answer 175)
Match the following and select correct option. Column I Column II A. B. C. D. Accessory duct Accessory glands Spermatids Primary spermatocytes 1. 2. 3. 4. 5 Seminal vesicles Vas deferens Uterus 23 chromosomes 46 chromosomes
A)
A-4,3 B-5 C-2 D-1
done
clear
B)
A-4,5 B-2 C-1 D-3
done
clear
C)
A-2,1 B-3 C-5 D-4
done
clear
D)
A-2,3 B-1 C-4 D-5
done
clear
View Answer play_arrow
question_answer 176) Caput epididymis is a narrow tubule present on the inner side of testis. It is present in between
A)
vasa efferentia and carpus epididymis
done
clear
B)
rete testis and vasa efferentia
done
clear
C)
vasa efferentia and rete testis
done
clear
D)
cauda epididymis and vas deferens
done
clear
View Answer play_arrow
question_answer 177) Compare the statements A and B. Statement A When the urine moves through the descending limb, it becomes hypertonic and as it passes through the ascending limb of Henles loop. It becomes hypotonic. Statement B. The descending limb is permeable to sodium ions, while the ascending limb is impermeable to sodium ions.
A)
Statement A is wrong and B is correct
done
clear
B)
Statement A is correct and B is wrong,
done
clear
C)
Both Statements A and B are wrong
done
clear
D)
Both Statements A and B are correct
done
clear
View Answer play_arrow
question_answer 178) The scapula is a large triangular flat bone situated in the dorsal part of the thorax between.
A)
Fifth and sixth rib
done
clear
B)
Second and fifth rib
done
clear
C)
Third and fourth rib
done
clear
D)
Second and seventh rib
done
clear
View Answer play_arrow
question_answer 179) The example of pivot joint is
A)
ankle joints
done
clear
B)
hip joints
done
clear
C)
radio-ulna joints
done
clear
D)
Metatarsophalangeal joints
done
clear
View Answer play_arrow
question_answer 180)
Read the following statements regarding muscle proteins. I. Actin is a thin filament and is made up of two f-actins. II. The complex protein, tropomyopin is distributed at regular intervals on the troponin. III. Myosin is a thick filament, which is also a polymerised protein. IV. The globular head of meromyosin consists of Light Meromyosin (LMM).
The above statements are correct.
A)
I, II and IV
done
clear
B)
I and III
done
clear
C)
I, II and III
done
clear
D)
II and IV
done
clear
View Answer play_arrow
question_answer 181) The third ventricle of the brain is situated in the
A)
roof of metencephalon
done
clear
B)
base of myelencephalon
done
clear
C)
roof of diencephalon
done
clear
D)
base of telencephalon
done
clear
View Answer play_arrow
question_answer 182) ..... accelerates heart beat due to stimulation of adrenal, medula by sympathetic nerves.
A)
Thyroxide
done
clear
B)
Vasopressin
done
clear
C)
Adrenaline
done
clear
D)
Thyroxine
done
clear
View Answer play_arrow
question_answer 183) MSH is secreted by
A)
middle lobe of pituitary
done
clear
B)
anterior lobe of pituitary
done
clear
C)
endostyle
done
clear
D)
posterior lobe P of pituitary
done
clear
View Answer play_arrow
question_answer 184) This trace element is needed for insulin to exert its maximal effect in glucose uptake.
A)
Chromium
done
clear
B)
Selenium
done
clear
C)
Vanadium
done
clear
D)
Molybdenum
done
clear
View Answer play_arrow
question_answer 185) The number of occipital condyles in man is/are
A)
two
done
clear
B)
one
done
clear
C)
four
done
clear
D)
three
done
clear
View Answer play_arrow
question_answer 186) Cereals during germination derive their food from
A)
soil
done
clear
B)
starch
done
clear
C)
embryo
done
clear
D)
aleurone grains
done
clear
View Answer play_arrow
question_answer 187) On the earth, the amount of carbon fixed by photosynthesis is nearly
A)
\[7\times {{10}^{13}}kg/yr\]
done
clear
B)
\[7\times {{10}^{13}}kg/yr\]
done
clear
C)
\[7\times {{10}^{10}}kg/yr\]
done
clear
D)
\[7\times {{10}^{13}}kg/yr\]
done
clear
View Answer play_arrow
question_answer 188) Which one of the following theories for ascent of sap was proposed by an eminent Indian scientist JC Bose?
A)
Relay pump theory
done
clear
B)
Pulsation theory
done
clear
C)
Root pressure theory
done
clear
D)
Transpiration pull theory
done
clear
View Answer play_arrow
question_answer 189)
Match the name of the scientists given under column I with their important contributions given under columb II. Choose the answer, which gives correct combination of the alphabets. Column I Column II A. Peter Mitchell 1. Law of (Smiting factor B. Blackmann 2. Dark reaction of photosynthesis C. Daniel Arnon 3. Photosynthetic phosphorylation D. Melvin Calvin 4. Chemiosmotic hypothesis
A)
A-4 B-1 C-3 D-2
done
clear
B)
A-2 B-3 C-1 D-4
done
clear
C)
A-1 B-3 C-4 D-2
done
clear
D)
A-1 B-4 C-2 D-3
done
clear
View Answer play_arrow
question_answer 190) In Electron Transport System (ETS), which of the following cytochrome reacts with oxygen?
A)
\[Cyt-{{a}_{3}}\]
done
clear
B)
\[Cyt-f\]
done
clear
C)
\[Cyt-b\]
done
clear
D)
\[Cyt-{{b}_{6}}\]
done
clear
View Answer play_arrow
question_answer 191) The number of NADPH molecules that are used during the conversion of carbon dioxide into one molecules of glucose.
A)
4
done
clear
B)
1
done
clear
C)
12
done
clear
D)
6
done
clear
View Answer play_arrow
question_answer 192) Which of the following is not a hereditary disease?
A)
Cretinism
done
clear
B)
Cystic fibrosis
done
clear
C)
Haemophilia
done
clear
D)
Thalassemia
done
clear
View Answer play_arrow
question_answer 193) Percentage of recombination between A and B is 9%, A and C is 17%, B and C is 26%, then the arrangement of genes is
A)
ACB
done
clear
B)
BAC
done
clear
C)
BCA
done
clear
D)
ABC
done
clear
View Answer play_arrow
question_answer 194) Phenotypic of Downs syndrome increases when the maternal age is
A)
3:.1
done
clear
B)
1 :1
done
clear
C)
1:2: 1
done
clear
D)
2: 1
done
clear
View Answer play_arrow
question_answer 195) The concept of one-gene-one enzyme was proposed by
A)
Leeuwenhoek
done
clear
B)
Watson and Crick
done
clear
C)
Carl Correns
done
clear
D)
Beadle and Tatum
done
clear
View Answer play_arrow
question_answer 196) The enzyme decarboxylase catalyses
A)
fumaric acid to malic acid
done
clear
B)
malic acid to oxaloacetic acid
done
clear
C)
conversion of citric acid to c/s aconitic acid
done
clear
D)
oxalosuccinic acid to \[\alpha -\]ketoglutaric acid
done
clear
View Answer play_arrow
question_answer 197) The loss of one single chromosome cretes a condition called
A)
haploid
done
clear
B)
nullisomy
done
clear
C)
monosomy
done
clear
D)
trisomy
done
clear
View Answer play_arrow
question_answer 198) Dental formula of human being is
A)
12\[l2,C1\,PM3,\,P3\]
done
clear
B)
\[l2,C2\,PM3,\,P1\]
done
clear
C)
\[l3,C1\,PM2,\,P3\]
done
clear
D)
\[l3,C2\,PM1,\,P3\]
done
clear
View Answer play_arrow
question_answer 199) Respiratory movements are under the control of
A)
medulla oblongata
done
clear
B)
carpus callosum
done
clear
C)
cerebral hemispheres
done
clear
D)
crura cerebri
done
clear
View Answer play_arrow
question_answer 200)
Identify the human development stage shown below as well as the related right place of its occurrence in a normal pregnant woman, and select the right option for the two, together.
A)
Late morula - Middle part of fallopian tube
done
clear
B)
Blastula - End part of fallopian tube
done
clear
C)
Blastocyst - Uterine wall
done
clear
D)
8-celled morula - Starting point of Fallopian tube
done
clear
View Answer play_arrow
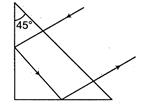
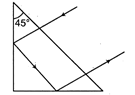
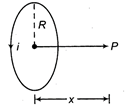
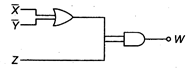
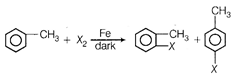
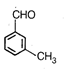
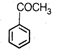
 Parent cell
Parent cell