A)
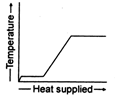
B)
C)
D)
Correct Answer: A
Solution :
The temperature of ice will first increases from \[-10{{\,}^{o}}C\]to\[0{{\,}^{o}}C.\] Heat supplied in this process will be \[{{Q}_{1}}=m{{s}_{i}}(10)\] Here, m = mass of ice. Si = specific heat of ice Then ice starts melting. Temperature during melting will remain constant \[(0{{\,}^{o}}C).\] Heat supplied in the process will be \[{{Q}_{2}}=mL\,L=\]latent heat of melting Now the temperature of water will increase from \[0{{\,}^{o}}C\]to \[{{100}^{o}}C.\] Heat supplied will be \[{{Q}_{3}}=m{{s}_{w}}(100){{s}_{w}}=\]specific heat of water Finally water at \[100{{\,}^{o}}C\]will be convened into steam at \[100{{\,}^{o}}C\]and during this process temperature again remains constant. Temperature versus heat supplied graph will be as follows:
You need to login to perform this action.
You will be redirected in
3 sec
