A) \[N{{H}_{3}}\]has larger molecular weight
B) \[N{{H}_{3}}\] undergoes umbrella inversion
C) \[N{{H}_{3}}\] forms hydrogen bond
D) \[N{{H}_{3}}\]contains ionic bonds whereas \[P{{H}_{3}}\]contains covalent bonds
Correct Answer: C
Solution :
Boiling point of ammonia is much higher than phosphine. It is due to extensive hydrogen bonding found in ammonia.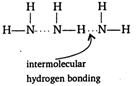
You need to login to perform this action.
You will be redirected in
3 sec
