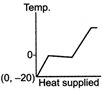
A)
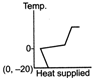
B)
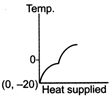
C)
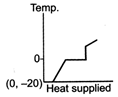
D)
Correct Answer: A
Solution :
The ice at \[-\text{20}{{\,}^{\text{o}}}\text{C}\]converts to ice at \[\text{0}{{\,}^{\text{o}}}\text{C,}\] thus having an increase in temperature. On further heating, ice at \[\text{0}{{\,}^{\text{o}}}\text{C}\]is converted into water at \[\text{0}{{\,}^{\text{o}}}\text{C,}\] thus the temperature remaining constant, a change of phase occurs. Water at \[\text{0}{{\,}^{\text{o}}}\text{C}\]when heated changes to water at \[\text{100}{{\,}^{\text{o}}}\text{C,}\] thus resulting in an increase of temperature. Water at \[\text{100}{{\,}^{\text{o}}}\text{C}\]changes to steam at \[\text{100}{{\,}^{\text{o}}}\text{C,}\] thus resulting in a change of phase. Hence, Fig. is the correct answer.You need to login to perform this action.
You will be redirected in
3 sec
