A) The backward reaction has higher activation energy than the forward reaction
B) The backward and the forward processes have the same activation energy
C) The backward reaction has lower activation energy
D) No activation energy is required at all since energy is liberated in the process
Correct Answer: A
Solution :
The energy profile diagram for a exothermic reaction is as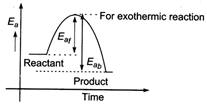 \[\therefore \] \[{{({{E}_{a}})}_{b}}>{{({{E}_{a}})}_{f}}\]
\[\therefore \] \[{{({{E}_{a}})}_{b}}>{{({{E}_{a}})}_{f}}\]
You need to login to perform this action.
You will be redirected in
3 sec
