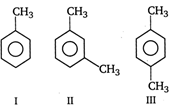
A) II = III = I
B) II > III > I
C) III > II > I
D) I = III > II
Correct Answer: B
Solution :
The ease of nitration of the hydrocarbons depends upon the stability of arenium ion formed.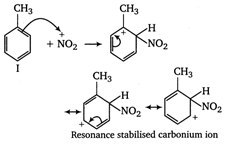
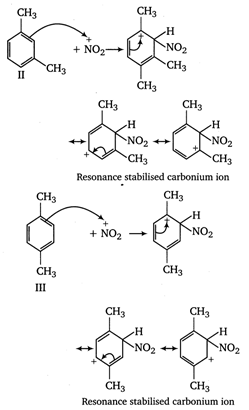 In compound II, the carbonium ion has two methyl groups (with \[+\,I\]effect) capable of partially neutralising the positive charges on the ring carbon atoms. In compound I and II, only one methyl group can act in this way. Moreover, in compoumd III two methyl groups has \[+\,I\]effet, whereas in compound I only one methyl group with \[+\,I\]effect is present. Thus, the stability of arenium ion and ease of nitration follows the order II > III > I
In compound II, the carbonium ion has two methyl groups (with \[+\,I\]effect) capable of partially neutralising the positive charges on the ring carbon atoms. In compound I and II, only one methyl group can act in this way. Moreover, in compoumd III two methyl groups has \[+\,I\]effet, whereas in compound I only one methyl group with \[+\,I\]effect is present. Thus, the stability of arenium ion and ease of nitration follows the order II > III > I
You need to login to perform this action.
You will be redirected in
3 sec
