A) m- nitrophenol>p-nitrophenol>o-nitrophenol
B) o-nitrophenol >m-nitrophenol> p-nitrophenol
C) p-nitrophenol>m-mtrophenol> o-nitrophenol
D) p-nitrophenol > o-nitrophenol > m-nitrophenol
Correct Answer: D
Solution :
\[-\text{N}{{\text{O}}_{\text{2}}}\]group at \[\text{o}-\]and p- position withdraws electrons of the O?H bond towards itself by the stronger -R effect while the \[-\text{N}{{\text{O}}_{\text{2}}}\] group at m-position with draws electrons of the O?H bond by the weaker -I effect. Thus, o- and p- nitrophenols are more acidic than m- nitrophenol. Among o- and p- nitrophenols, o- nitrophenol is little less acidic than p-nitrophenol due to intramolecular H-bonding which makes loss of a proton little more difficult.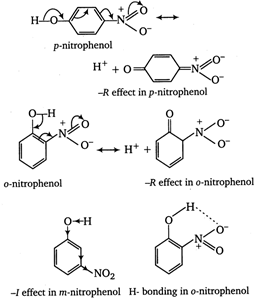 \[\therefore \]The order of acidity of nitrophenols is p- nitrophenol > o-nitrophenol > m-nitrophenol
\[\therefore \]The order of acidity of nitrophenols is p- nitrophenol > o-nitrophenol > m-nitrophenol
You need to login to perform this action.
You will be redirected in
3 sec
