A) \[sp,0\]
B) \[s{{p}^{2}},0\]
C) \[s{{p}^{3}},0\]
D) \[ds{{p}^{2}},1\]
Correct Answer: C
Solution :
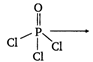 \[4\sigma \]bonds\[\Rightarrow s{{p}^{3}}\] hybridisation without lone pair of electrons.
\[4\sigma \]bonds\[\Rightarrow s{{p}^{3}}\] hybridisation without lone pair of electrons.
You need to login to perform this action.
You will be redirected in
3 sec
