A) \[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-O-C{{H}_{3}}\]
B) \[C{{H}_{3}}-C{{H}_{2}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-O-C{{H}_{3}}\]
C) \[C{{H}_{3}}-\underset{C{{H}_{3}}}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-O-C{{H}_{3}}\]
D) \[C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-C{{H}_{2}}-O-C{{H}_{3}}\]
Correct Answer: C
Solution :
The ether, which gives more stable carbocation, gives \[C{{H}_{3}}OH\]as one of the product with hot concentrated HI. The order of stability of carbocation is \[{{3}^{o}}>{{2}^{o}}>{{1}^{o}}\] Thus,\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\overset{C{{H}_{3}}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-OC{{H}_{3}}\] gives\[C{{H}_{3}}OH\] as one of the reaction. The reaction proceeds as \[{{H}_{3}}C-\underset{C{{H}_{3}}}{\overset{CH3}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-O-C{{H}_{3}}+{{H}^{+}}\]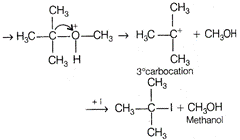
You need to login to perform this action.
You will be redirected in
3 sec
