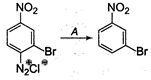
 A is
A is
A) \[HgS{{O}_{4}}/{{H}_{2}}S{{O}_{4}}\]
B) \[C{{u}_{2}}C{{l}_{2}}\]
C) \[{{H}_{3}}P{{O}_{2}}\]and \[{{H}_{2}}O\]
D) \[{{H}^{+}}/{{H}_{2}}O\]
Correct Answer: C
Solution :
When diazonium salt is treated with \[{{H}_{3}}P{{O}_{2}}\]followed by hydrolysis, it diazonium group is replaced by -H resulting to the formation of hydrocarbon. Thus, A must be \[{{H}_{3}}P{{O}_{2}}/{{H}_{2}}O.\]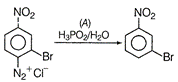
You need to login to perform this action.
You will be redirected in
3 sec
