A) large size of F compared to H
B) large size of N compared to F
C) opposite polarity of N in the two molecules
D) small size of H compared to N
Correct Answer: C
Solution :
In \[N{{F}_{3}},N\]is less electronegative as compared to F but in \[\text{N}{{\text{H}}_{\text{3}}}\text{.}\]it is more electronegative than H And in case of same central atom, as the electronegativity of other atoms increases bond angle decreases. Thus, bond angle in \[\text{N}{{\text{F}}_{\text{3}}}\]Is smaller than that in NH3 because of the difference in the polarity of N in these molecules.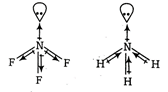
You need to login to perform this action.
You will be redirected in
3 sec
