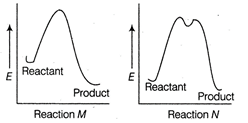
A) Reaction M is faster and less exothermic than reaction N
B) Reaction M is slower and less exothermic than reaction N
C) Reaction M is faster and more exothermic than reaction N
D) Reaction M is slower and more exothermic than reaction N
Correct Answer: C
Solution :
In reaction M. there is only an intermediate step, so it is a fast reaction. Further, in reaction M energy of product is much lesser than that of the reactant, as compared to as shown in reaction N. Thus, reaction M is faster and more exothermic than reaction N.You need to login to perform this action.
You will be redirected in
3 sec
