Answer:
(a) Since, the element lies
in group 2, it must be an alkaline earth metal. Since, it lies in the third
period, it must be magnesium (Mg).
(b) Atomic number of
Mg is 12, therefore, its electronic configuration is:![]() (c) When Mg
burns in the presence of air it forms a basic oxide, MgO.
(c) When Mg
burns in the presence of air it forms a basic oxide, MgO.
![]() (d) When MgO
is dissolved in water it forms magnesium hydroxide
(d) When MgO
is dissolved in water it forms magnesium hydroxide
![]() (e) Mg has 2
valence electrons [as electronic configuration of
(e) Mg has 2
valence electrons [as electronic configuration of![]() ]
oxygen has6 valence electron [as electronic configuration of
]
oxygen has6 valence electron [as electronic configuration of![]() ].
Electron dot
structure for the formation of magnesium oxide.
].
Electron dot
structure for the formation of magnesium oxide.
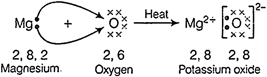
You need to login to perform this action.
You will be redirected in
3 sec
