Answer:
Although butane and propanol have comparable
molecularmasses (58 and 60 respectively) but, in propanol, polar ?OH group
ispresent, due to which strong intermolecular hydrogen bonding existsbetween
its molecules. Whereas in butane weak van der Waals' forcesof attraction are
the only forces between the molecules. Therefore,propanol has higher boiling
point (391 K) as compared to that of butane(309 K).
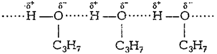
You need to login to perform this action.
You will be redirected in
3 sec
