Answer:
In aryl alkyl ethers, +R effect of the alkoxy group ![]() increases
the electron density in the benzene ring thereby activatingthe benzene ring
towards electrophilic substitution reactions.
increases
the electron density in the benzene ring thereby activatingthe benzene ring
towards electrophilic substitution reactions.
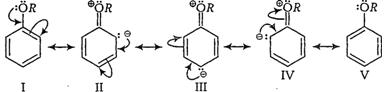 Electron density is more at o-
and p-positions so o- and p-products aremainly formed during electrophilic
substitution reactions.
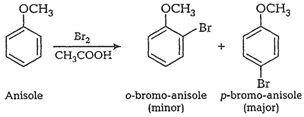
e.g.,
Electron density is more at o-
and p-positions so o- and p-products aremainly formed during electrophilic
substitution reactions.
e.g.,
You need to login to perform this action.
You will be redirected in
3 sec
