Answer:
(i)
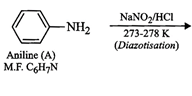
Since compound A ![]() is sparingly
soluble in water and gives a water soluble substance (B), on treatment with
mineral acid, therefore, compound (A) must be an amine.
(ii) Since compound (A) with m.p.
is sparingly
soluble in water and gives a water soluble substance (B), on treatment with
mineral acid, therefore, compound (A) must be an amine.
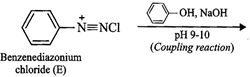
(ii) Since compound (A) with m.p. ![]() reacts
with
reacts
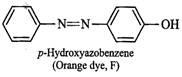
with ![]() to form a compound (E) which
reacts with phenol in alkaline medium to give an orange dye (F), therefore,
compound (A) must be a aromatic
to form a compound (E) which
reacts with phenol in alkaline medium to give an orange dye (F), therefore,
compound (A) must be a aromatic![]() amine,
i.e., aniline,
amine,
i.e., aniline, ![]() . If A is aniline,
then E must be benzenediazonium chloride and the orange dye (F) must be
p-hydroxyazobenzene.
. If A is aniline,
then E must be benzenediazonium chloride and the orange dye (F) must be
p-hydroxyazobenzene.
 (iii) Since compound (A), i.e., aniline reacts
(iii) Since compound (A), i.e., aniline reacts ![]() in presence of alcoholic KOH
to form a compound (C) having obnoxious smell, therefore, compound (C) must be
benzene isonitrile.
in presence of alcoholic KOH
to form a compound (C) having obnoxious smell, therefore, compound (C) must be
benzene isonitrile.
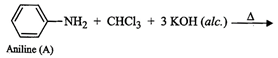
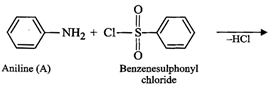
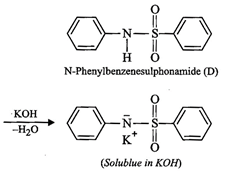
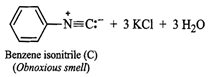 (iv) Since compound (A), i.e., aniline reacts with
benzenesulphonyl chloride to form compound (D) which is soluble in alkali,
therefore, (D) must be N-phenylbenzenesulphonamide.
(iv) Since compound (A), i.e., aniline reacts with
benzenesulphonyl chloride to form compound (D) which is soluble in alkali,
therefore, (D) must be N-phenylbenzenesulphonamide.
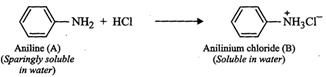
 (vi) Since compound (A), i.e., aniline reacts with mineral
acids (say HCl) to form water soluble en (B), therefore, (B) must be anilinium
chloride
(vi) Since compound (A), i.e., aniline reacts with mineral
acids (say HCl) to form water soluble en (B), therefore, (B) must be anilinium
chloride
You need to login to perform this action.
You will be redirected in
3 sec
