Answer:
Rate of any elementary reaction can be represented
as
![]() After changing concentration to
its triple value A = 3A f becomes 27 r
After changing concentration to
its triple value A = 3A f becomes 27 r
![]()
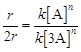
![]() Hence, n= 3
Order of reaction is three.
Hence, n= 3
Order of reaction is three.
You need to login to perform this action.
You will be redirected in
3 sec
