Answer:
Soap molecule can be represented as
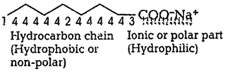 Hydrocarbon chain is water
repelling and ionic part is water attracting.When soap is dissolved in water
and a dirty cloth is agitated in thesolution, the oily dirt attaches to the
hydrocarbon part while waterattaches to the ionic part.
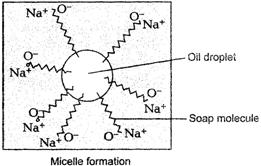
Now these soap molecules arrange
themselves in the form of micelles.
As the mixture is agitated more,
the more and more dirt particles leavethe cloth and get attached to the soap
molecules. The negative chargesof micelles prevent the dirt to form aggregates.
Thus, soap removes dirtby reducing the surface tension of water.
Hydrocarbon chain is water
repelling and ionic part is water attracting.When soap is dissolved in water
and a dirty cloth is agitated in thesolution, the oily dirt attaches to the
hydrocarbon part while waterattaches to the ionic part.
Now these soap molecules arrange
themselves in the form of micelles.
As the mixture is agitated more,
the more and more dirt particles leavethe cloth and get attached to the soap
molecules. The negative chargesof micelles prevent the dirt to form aggregates.
Thus, soap removes dirtby reducing the surface tension of water.
You need to login to perform this action.
You will be redirected in
3 sec
