Answer:
(i) See answer to Q.25?SATQ
of CBSE examination questions.
(ii) In [FeF6]3?,
F? is a weak ligand and cannot cause pairing in Fe3+. So
the other nd orbitals are involved in hybridization (sp3d2).
The complex has octahedral geometry. It is paramagnetic in nature due to the presence
of five unpaired electrons.
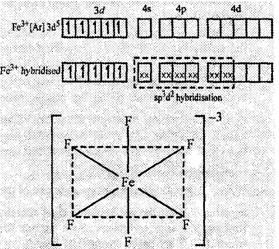 Octahedral
structure of [FeF6]3?
(iii) Try yourself
(iv) See answer to
Q. 49 of Additional Important questions.
Octahedral
structure of [FeF6]3?
(iii) Try yourself
(iv) See answer to
Q. 49 of Additional Important questions.
You need to login to perform this action.
You will be redirected in
3 sec
