Answer:
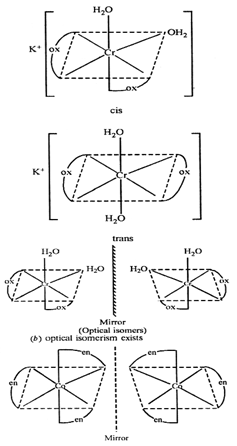
(a) (i) Both
geometrical and optical isomers can exist.
 (c)Isonisationisomers
:
[Co(NH3)5
(NO2)] (NO3)2
[Co(NH3)5
(NO3)] (NO2)(NO3)
Linkage isomers :
[Co(NH3)5
(NO2)] (NO3)2
[Co(NH3)5
(ONO)] (NO3)2
Octahedral
complexes of the type [MA5B] do not show geometrical and optical
isomerism.
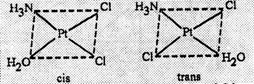
(d) Geometrical
isomers (cis and trans)
(c)Isonisationisomers
:
[Co(NH3)5
(NO2)] (NO3)2
[Co(NH3)5
(NO3)] (NO2)(NO3)
Linkage isomers :
[Co(NH3)5
(NO2)] (NO3)2
[Co(NH3)5
(ONO)] (NO3)2
Octahedral
complexes of the type [MA5B] do not show geometrical and optical
isomerism.
(d) Geometrical
isomers (cis and trans)
You need to login to perform this action.
You will be redirected in
3 sec
