Answer:
Bonding in metal
carbonyls.
The formation of bonds between the metal and carbon atom carbon monoxide is as
described below:
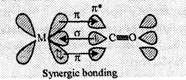
Sigma-overlap involving donation
of lone-pairof electrons on carbon of CO into vacant d-orbital on the metal to
form ![]()
![]() bond.
(ii) Pi-overlap involving donation
of electrons from a filled metal d-orbital of metal into a vacant anti-bonding
bond.
(ii) Pi-overlap involving donation
of electrons from a filled metal d-orbital of metal into a vacant anti-bonding ![]() orbital
of CO forming
M
orbital
of CO forming
M![]() CO
CO![]() -bond.
-bond.
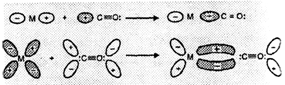 Thus, M to C bond
in metal carbonyls consists of a weak C
Thus, M to C bond
in metal carbonyls consists of a weak C ![]() M
sigma bond and M
M
sigma bond and M ![]() C pi
bond which results due to back-bonding. The back bonding between metal and
carbon atom creates a synergic effect which stabilises the M to C bond,
C pi
bond which results due to back-bonding. The back bonding between metal and
carbon atom creates a synergic effect which stabilises the M to C bond,
You need to login to perform this action.
You will be redirected in
3 sec
