Answer:
(a,
c)
Molecular orbital electronic configuration of![]() in
in ![]() is
is
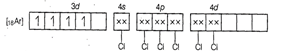 Number of unpaired electrons = 4
Magnetic property = Paramagnetic
Molecular orbital electronic configuration of
Number of unpaired electrons = 4
Magnetic property = Paramagnetic
Molecular orbital electronic configuration of ![]() in
in ![]() is
is
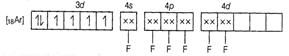 Number of unpaired electrons = 4
Magnetic property = Paramagnetic
Molecular orbital electronic configuration of
Number of unpaired electrons = 4
Magnetic property = Paramagnetic
Molecular orbital electronic configuration of ![]() in
in ![]() is
is
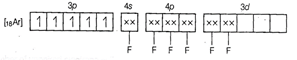 Number of unpaired electrons = 5
Magnetic property = Paramagnetic
Molecular orbital electronic configuration of
Number of unpaired electrons = 5
Magnetic property = Paramagnetic
Molecular orbital electronic configuration of ![]() in
in ![]() is
is
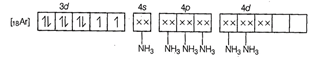 Number of unpaired electrons =2.
Magnetic property = Paramagnetic
Thus,
Number of unpaired electrons =2.
Magnetic property = Paramagnetic
Thus, ![]() and
and ![]() are paramagnetic having four
electrons each.
Hence, correct choices are (a) and (c).
are paramagnetic having four
electrons each.
Hence, correct choices are (a) and (c).
You need to login to perform this action.
You will be redirected in
3 sec
