Answer:
With
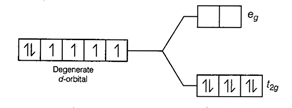
weak field ligands; ![]() < P, (pairing
energy) so, the electronic configuration of Co (III) will be
< P, (pairing
energy) so, the electronic configuration of Co (III) will be ![]() i.e., it has 4 unpaired
electrons and is paramagnetic.
i.e., it has 4 unpaired
electrons and is paramagnetic.
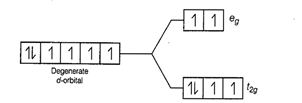 With strong field ligands,
With strong field ligands, ![]() >
P (pairing energy), so pairing occurs thus, the electronic configuration will
be
>
P (pairing energy), so pairing occurs thus, the electronic configuration will
be![]() . It has no unpaired electrons
and is diamagnetic.
. It has no unpaired electrons
and is diamagnetic.
You need to login to perform this action.
You will be redirected in
3 sec
