Answer:
As
we know, ![]() where,
where, ![]() =
magnetic moment
=
magnetic moment
![]() = number of
unpaired electrons
It
= number of
unpaired electrons
It ![]() =
1.74 i.e., n = 1
and
=
1.74 i.e., n = 1
and ![]() =
5.92 i.e., n = 5 .
=
5.92 i.e., n = 5 .
![]() involves
involves ![]() hybridisation with one
unpaired electron (as shown by its magnetic moment 1.74 BM) and
hybridisation with one
unpaired electron (as shown by its magnetic moment 1.74 BM) and ![]() involves
involves ![]() hybridisation with five unpaired
electrons (because magnetic moment equal to 5.92 BM).
hybridisation with five unpaired
electrons (because magnetic moment equal to 5.92 BM).
![]() is stronger ligand
than
is stronger ligand
than ![]() according to spectrochemical
series.
according to spectrochemical
series. ![]() >
P for
>
P for ![]() hence,
fourth electron will pair itself. Whereas for water pairing
will not happen for
hence,
fourth electron will pair itself. Whereas for water pairing
will not happen for ![]() the electronic
configuration of
the electronic
configuration of ![]() is
is
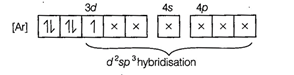 One unpaired electron
For
One unpaired electron
For ![]() the
electronic configuration of
the
electronic configuration of ![]() is
is
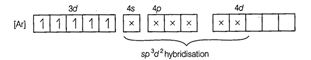 Five unpaired electron
Hence,
Five unpaired electron
Hence, ![]() and
and ![]() are inner orbital and outer
orbital complex respectively
are inner orbital and outer
orbital complex respectively
You need to login to perform this action.
You will be redirected in
3 sec
